Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131
- PMID: 17145787
- PMCID: PMC1797775
- DOI: 10.1128/AAC.00968-06
Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131
Abstract
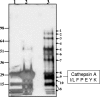
GS-7340 and GS-9131 {9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]-propyl]adenine and 9-(R)-4'-(R)-[[[(S)-1-[(ethoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]-2'-fluoro-1'-furanyladenine, respectively} are novel alkylalaninyl phenyl ester prodrugs of tenofovir {9-R-[(2-phosphonomethoxy)propyl]adenine} (TFV) and a cyclic nucleotide analog, GS-9148 (phosphonomethoxy-2'-fluoro-2', 3'-dideoxydidehydroadenosine), respectively. Both prodrugs exhibit potent antiretroviral activity against both wild-type and drug-resistant human immunodeficiency virus type 1 strains and excellent in vivo pharmacokinetic properties. In this study, the main enzymatic activity responsible for the initial step in the intracellular activation of GS-7340 and GS-9131 was isolated from human peripheral blood mononuclear cells and identified as lysosomal carboxypeptidase A (cathepsin A [CatA]; EC 3.4.16.5). Biochemical properties of the purified hydrolase (native complex and catalytic subunit molecular masses of 100 and 29 kDa, respectively; isoelectric point [pI] of 5.5) matched those of CatA. Recombinant CatA and the isolated prodrug hydrolase displayed identical susceptibilities to inhibitors and identical substrate preferences towards a panel of tenofovir phosphonoamidate prodrugs. Incubation of both enzymes with 14C-labeled GS-7340 or [3H]difluorophosphonate resulted in the covalent labeling of identical 29-kDa catalytic subunits. Finally, following a 4-h incubation with GS-7340 and GS-9131, the intracellular concentrations of prodrug metabolites detected in CatA-negative fibroblasts were approximately 7.5- and 3-fold lower, respectively, than those detected in normal control fibroblasts. Collectively, these data demonstrate the key role of CatA in the intracellular activation of nucleotide phosphonoamidate prodrugs and open new possibilities for further improvement of this important class of antiviral prodrugs.
Figures
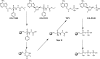
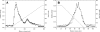

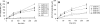
Similar articles
-
Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases.Mol Pharmacol. 2008 Jul;74(1):92-100. doi: 10.1124/mol.108.045526. Epub 2008 Apr 22. Mol Pharmacol. 2008. PMID: 18430788
-
Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors.Antimicrob Agents Chemother. 2015 Oct 26;60(1):316-22. doi: 10.1128/AAC.01834-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26503655 Free PMC article.
-
Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131.Antimicrob Agents Chemother. 2008 Feb;52(2):655-65. doi: 10.1128/AAC.01215-07. Epub 2007 Dec 3. Antimicrob Agents Chemother. 2008. PMID: 18056282 Free PMC article.
-
Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus.Antiviral Res. 2016 Jan;125:63-70. doi: 10.1016/j.antiviral.2015.11.009. Epub 2015 Nov 27. Antiviral Res. 2016. PMID: 26640223 Review.
-
Amino Acid Ester Prodrugs of Nucleoside and Nucleotide Antivirals.Mini Rev Med Chem. 2017;17(10):818-833. doi: 10.2174/1389557517666170216151601. Mini Rev Med Chem. 2017. PMID: 28215138 Review.
Cited by
-
The ProTides Boom.ChemMedChem. 2016 Jun 6;11(11):1114-6. doi: 10.1002/cmdc.201600156. Epub 2016 May 9. ChemMedChem. 2016. PMID: 27159529 Free PMC article.
-
ProTides of BVdU as potential anticancer agents upon efficient intracellular delivery of their activated metabolites.Bioorg Med Chem Lett. 2016 Dec 1;26(23):5618-5623. doi: 10.1016/j.bmcl.2016.10.077. Epub 2016 Oct 27. Bioorg Med Chem Lett. 2016. PMID: 27818111 Free PMC article.
-
A long acting nanoformulated lamivudine ProTide.Biomaterials. 2019 Dec;223:119476. doi: 10.1016/j.biomaterials.2019.119476. Epub 2019 Sep 5. Biomaterials. 2019. PMID: 31525692 Free PMC article.
-
Effects of Genetic Polymorphisms of Cathepsin A on Metabolism of Tenofovir Alafenamide.Genes (Basel). 2021 Dec 20;12(12):2026. doi: 10.3390/genes12122026. Genes (Basel). 2021. PMID: 34946974 Free PMC article.
-
Tenofovir alafenamide use in pregnant and lactating women living with HIV.Expert Opin Drug Metab Toxicol. 2020 Apr;16(4):333-342. doi: 10.1080/17425255.2020.1738384. Epub 2020 Mar 17. Expert Opin Drug Metab Toxicol. 2020. PMID: 32125906 Free PMC article. Review.
References
-
- Antoniou, T., L. Y. Park-Wyllie, and A. L. Tseng. 2003. Tenofovir: a nucleotide analog for the management of human immunodeficiency virus infection. Pharmacotherapy 23:29-43. - PubMed
-
- Balzarini, J. 1994. Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16:113-126. - PubMed
-
- Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

