Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy
- PMID: 17145798
- PMCID: PMC1797767
- DOI: 10.1128/AAC.00414-06
Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy
Abstract
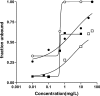
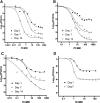
Members of the fluoroquinolone class are being actively evaluated for inclusion in tuberculosis chemotherapy regimens, and we sought to determine the best in vitro and pharmacodynamic predictors of in vivo efficacy in mice. MICs for Mycobacterium tuberculosis H37Rv were 0.1 mg/liter (sparfloxacin [SPX]) and 0.5 mg/liter (moxifloxacin [MXF], ciprofloxacin [CIP], and ofloxacin [OFX]). The unbound fraction in the presence of murine serum was concentration dependent for MXF, OFX, SPX, and CIP. In vitro time-kill studies revealed a time-dependent effect, with the CFU reduction on day 7 similar for all four drugs. However, with a J774A.1 murine macrophage tuberculosis infection model, CIP was ineffective at up to 32x MIC. In addition, MXF, OFX, and SPX exhibited less activity than had been seen in the in vitro time-kill study. After demonstrating that the area under the concentration-time curve (AUC) and maximum concentration of drug in plasma were proportional to the dose in vivo, dose fractionation studies with total oral doses of 37.5 to 19,200 mg/kg of body weight (MXF), 225 to 115,200 mg/kg (OFX), 30 to 50,000 mg/kg (SPX), and 38 to 100,000 mg/kg (CIP) were performed with a murine aerosol infection model. MXF was the most efficacious agent (3.0+/-0.2 log10 CFU/lung reduction), followed by SPX (1.4+/-0.1) and OFX (1.5+/-0.1). CIP showed no effect. The ratio of the AUC to the MIC was the pharmacodynamic parameter that best described the in vivo efficacy. In summary, a lack of intracellular killing predicted the lack of in vivo activity of CIP. The in vivo rank order for maximal efficacy of the three active fluoroquinolones was not clearly predicted by the in vitro assays, however.
Figures



Similar articles
-
Activity of sitafloxacin against extracellular and intracellular Staphylococcus aureus in vitro and in vivo: comparison with levofloxacin and moxifloxacin.J Antibiot (Tokyo). 2012 May;65(5):229-36. doi: 10.1038/ja.2012.7. Epub 2012 Feb 15. J Antibiot (Tokyo). 2012. PMID: 22334239
-
Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling.J Infect Dis. 2004 Nov 1;190(9):1642-51. doi: 10.1086/424849. Epub 2004 Sep 24. J Infect Dis. 2004. PMID: 15478070
-
Evaluation of moxifloxacin, ciprofloxacin, gatifloxacin, ofloxacin, and levofloxacin concentrations in human conjunctival tissue.Arch Ophthalmol. 2005 Sep;123(9):1282-3. doi: 10.1001/archopht.123.9.1282. Arch Ophthalmol. 2005. PMID: 16157821 No abstract available.
-
Efficacy of fluoroquinolones against pathogenic oral bacteria.Mini Rev Med Chem. 2009 Sep;9(10):1147-58. doi: 10.2174/138955709789055243. Mini Rev Med Chem. 2009. PMID: 19534690 Review.
-
Minimal inhibitory concentrations of rifabutin, ciprofloxacin, and ofloxacin against Mycobacterium tuberculosis isolated before treatment of patients in Taiwan.Am Rev Respir Dis. 1989 Oct;140(4):987-9. doi: 10.1164/ajrccm/140.4.987. Am Rev Respir Dis. 1989. PMID: 2552883 Review.
Cited by
-
Building Optimal Three-Drug Combination Chemotherapy Regimens.Antimicrob Agents Chemother. 2020 Oct 20;64(11):e01610-20. doi: 10.1128/AAC.01610-20. Print 2020 Oct 20. Antimicrob Agents Chemother. 2020. PMID: 32900682 Free PMC article.
-
Ethambutol optimal clinical dose and susceptibility breakpoint identification by use of a novel pharmacokinetic-pharmacodynamic model of disseminated intracellular Mycobacterium avium.Antimicrob Agents Chemother. 2010 May;54(5):1728-33. doi: 10.1128/AAC.01355-09. Epub 2010 Mar 15. Antimicrob Agents Chemother. 2010. PMID: 20231389 Free PMC article.
-
Moxifloxacin-Mediated Killing of Mycobacterium tuberculosis Involves Respiratory Downshift, Reductive Stress, and Accumulation of Reactive Oxygen Species.Antimicrob Agents Chemother. 2022 Sep 20;66(9):e0059222. doi: 10.1128/aac.00592-22. Epub 2022 Aug 17. Antimicrob Agents Chemother. 2022. PMID: 35975988 Free PMC article.
-
Trimethoprim/Sulfamethoxazole and Moxifloxacin Therapy for a Pediatric Stenotrophomonas Maltophilia Ventriculoperitoneal Shunt Infection.J Pediatr Pharmacol Ther. 2019 Jan-Feb;24(1):61-65. doi: 10.5863/1551-6776-24.1.61. J Pediatr Pharmacol Ther. 2019. PMID: 30837817 Free PMC article.
-
A computational tool integrating host immunity with antibiotic dynamics to study tuberculosis treatment.J Theor Biol. 2015 Feb 21;367:166-179. doi: 10.1016/j.jtbi.2014.11.021. Epub 2014 Dec 9. J Theor Biol. 2015. PMID: 25497475 Free PMC article.
References
-
- Bryskier, A., and J. Lowther. 2002. Fluoroquinolones and tuberculosis. Expert Opin. Investig. Drugs 11:223-258. - PubMed
-
- Carryn, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095-2103. - PMC - PubMed
-
- Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

