Modification of the structure of peptidoglycan is a strategy to avoid detection by nucleotide-binding oligomerization domain protein 1
- PMID: 17145940
- PMCID: PMC1828529
- DOI: 10.1128/IAI.01597-06
Modification of the structure of peptidoglycan is a strategy to avoid detection by nucleotide-binding oligomerization domain protein 1
Abstract
Nucleotide-binding oligomerization domain (NOD) protein 1 (NOD1) and NOD2 are pathogen recognition receptors that sense breakdown products of peptidoglycan (PGN) (muropeptides). It is shown that a number of these muropeptides can induce tumor necrosis factor alpha (TNF-alpha) gene expression without significant TNF-alpha translation. This translation block is lifted when the muropeptides are coincubated with lipopolysaccharide (LPS), thereby accounting for an apparently synergistic effect of the muropeptides with LPS on TNF-alpha protein production. The compounds that induced synergistic effects were also able to activate NF-kappaB in a NOD1- or NOD2-dependent manner, implicating these proteins in synergistic TNF-alpha secretion. It was found that a diaminopimelic acid (DAP)-containing muramyl tetrapeptide could activate NF-kappaB in a NOD1-dependent manner, demonstrating that an exposed DAP is not essential for NOD1 sensing. The activity was lost when the alpha-carboxylic acid of iso-glutamic acid was modified as an amide. However, agonists of NOD2, such as muramyl dipeptide and lysine-containing muramyl tripeptides, were not affected by amidation of the alpha-carboxylic acid of iso-glutamic acid. Many pathogens modify the alpha-carboxylic acid of iso-glutamic acid of PGN, and thus it appears this is a strategy to avoid recognition by the host innate immune system. This type of immune evasion is in particular relevant for NOD1.
Figures



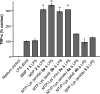

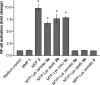

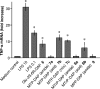

Similar articles
-
The Dual NOD1/NOD2 Agonism of Muropeptides Containing a Meso-Diaminopimelic Acid Residue.PLoS One. 2016 Aug 11;11(8):e0160784. doi: 10.1371/journal.pone.0160784. eCollection 2016. PLoS One. 2016. PMID: 27513337 Free PMC article.
-
Synthesis and proinflammatory properties of muramyl tripeptides containing lysine and diaminopimelic acid moieties.Chembiochem. 2005 Nov;6(11):2088-97. doi: 10.1002/cbic.200500181. Chembiochem. 2005. PMID: 16222728
-
Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture.Cell Microbiol. 2005 Jan;7(1):53-61. doi: 10.1111/j.1462-5822.2004.00433.x. Cell Microbiol. 2005. PMID: 15617523
-
NOD1 and NOD2: Molecular targets in prevention and treatment of infectious diseases.Int Immunopharmacol. 2018 Jan;54:385-400. doi: 10.1016/j.intimp.2017.11.036. Epub 2017 Dec 5. Int Immunopharmacol. 2018. PMID: 29207344 Review.
-
Chemical synthesis of peptidoglycan fragments for elucidation of the immunostimulating mechanism.J Endotoxin Res. 2007;13(3):189-96. doi: 10.1177/0968051907080739. J Endotoxin Res. 2007. PMID: 17621561 Review.
Cited by
-
The alternative translational profile that underlies the immune-evasive state of persistence in Chlamydiaceae exploits differential tryptophan contents of the protein repertoire.Microbiol Mol Biol Rev. 2012 Jun;76(2):405-43. doi: 10.1128/MMBR.05013-11. Microbiol Mol Biol Rev. 2012. PMID: 22688818 Free PMC article. Review.
-
Modulation of the NOD-like receptors NOD1 and NOD2: A chemist's perspective.Bioorg Med Chem Lett. 2019 May 15;29(10):1153-1161. doi: 10.1016/j.bmcl.2019.03.010. Epub 2019 Mar 8. Bioorg Med Chem Lett. 2019. PMID: 30890292 Free PMC article. Review.
-
Analysis of cell wall constituents of biocide-resistant isolates from dental-unit water line biofilms.Curr Microbiol. 2008 Oct;57(4):340-7. doi: 10.1007/s00284-008-9200-2. Curr Microbiol. 2008. PMID: 18661180
-
Membrane Association Dictates Ligand Specificity for the Innate Immune Receptor NOD2.ACS Chem Biol. 2017 Aug 18;12(8):2216-2224. doi: 10.1021/acschembio.7b00469. Epub 2017 Jul 25. ACS Chem Biol. 2017. PMID: 28708377 Free PMC article.
-
Plant peptidoglycan precursor biosynthesis: Conservation between moss chloroplasts and Gram-negative bacteria.Plant Physiol. 2022 Aug 29;190(1):165-179. doi: 10.1093/plphys/kiac176. Plant Physiol. 2022. PMID: 35471580 Free PMC article.
References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Beutler, B. 2004. Innate immunity: an overview. Mol. Immunol. 40:845-859. - PubMed
-
- Boneca, I. G. 2005. The role of peptidoglycan in pathogenesis. Curr. Opin. Microbiol. 8:46-53. - PubMed
-
- Bugg, T. D. H. 1999. Bacterial peptidoglycan. Biosynthesis and its inhibition, p. 241. In B. M. Pinto (ed.), Comprehensive natural products chemistry. Carbohydrates and their derivatives including tannins, cellulose, and related lignins, vol. 3. Elsevier, Amsterdam, The Netherlands.
-
- Caroff, M., D. Karibian, J. M. Cavaillon, and N. Haeffner-Cavaillon. 2002. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect. 4:915-926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

