Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement
- PMID: 17145958
- PMCID: PMC2118178
- DOI: 10.1084/jem.20061884
Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement
Abstract
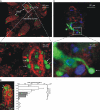
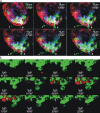
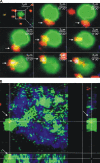
Cells lining the gastrointestinal tract serve as both a barrier to and a pathway for infectious agent entry. Dendritic cells (DCs) present in the lamina propria under the columnar villus epithelium of the small bowel extend processes across this epithelium and capture bacteria, but previous studies provided limited information on the nature of the stimuli, receptors, and signaling events involved in promoting this phenomenon. Here, we use immunohistochemical as well as dynamic explant and intravital two-photon imaging to investigate this issue. Analysis of CD11c-enhanced green fluorescent protein (EGFP) or major histocompatibility complex CII-EGFP mice revealed that the number of trans-epithelial DC extensions, many with an unusual "balloon" shape, varies along the length of the small bowel. High numbers of such extensions were found in the proximal jejunum, but only a few were present in the terminal ileum. The extensions in the terminal ileum markedly increased upon the introduction of invasive or noninvasive Salmonella organisms, and chimeric mouse studies revealed the key role of MyD88-dependent Toll-like receptor (TLR) signaling by nonhematopoietic (epithelial) elements in the DC extension response. Collectively, these findings support a model in which epithelial cell TLR signaling upon exposure to microbial stimuli induces active DC sampling of the gut lumen at sites distant from organized lymphoid tissues.
Figures
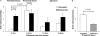



References
-
- Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. - PubMed
-
- Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. - PubMed
-
- Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710–720. - PubMed
-
- Lamm, M.E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311–340. - PubMed
-
- Macpherson, A.J., D. Gatto, E. Sainsbury, G.R. Harriman, H. Hengartner, and R.M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 288:2222–2226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

