BRCA1 foci in normal S-phase nuclei are linked to interphase centromeres and replication of pericentric heterochromatin
- PMID: 17145961
- PMCID: PMC2064668
- DOI: 10.1083/jcb.200602055
BRCA1 foci in normal S-phase nuclei are linked to interphase centromeres and replication of pericentric heterochromatin
Abstract
Breast cancer-associated protein 1 (BRCA1) forms foci at sites of induced DNA damage, but any significance of these normal S-phase foci is unknown. BRCA1 distribution does not simply mirror or overlap that of replicating DNA; however, BRCA1 foci frequently abut sites of BrdU incorporation, mostly at mid-to-late S phase. Although BRCA1 does not overlap XIST RNA across the inactive X chromosome, BRCA1 foci position overwhelmingly in heterochromatic regions, particularly the nucleolar periphery where many centromeres reside. In humans and mice, including early embryonic cells, BRCA1 commonly associates with interphase centromere-kinetochore complexes, including pericentric heterochromatin. Proliferating cell nuclear antigen or BrdU labeling demonstrates that BRCA1 localizes adjacent to, or "paints," major satellite blocks as chromocenters replicate, where topoisomerase is also enriched. BRCA1 loss is often associated with proliferative defects, including postmitotic bridges enriched with satellite DNA. These findings implicate BRCA1 in replication-linked maintenance of centric/pericentric heterochromatin and suggest a novel means whereby BRCA1 loss may contribute to genomic instability and cancer.
Figures
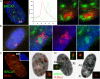



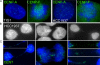
Similar articles
-
BRCA1 associates with the inactive X chromosome in late S-phase, coupled with transient H2AX phosphorylation.Chromosoma. 2005 Dec;114(6):432-9. doi: 10.1007/s00412-005-0029-1. Epub 2005 Nov 15. Chromosoma. 2005. PMID: 16240122
-
BRCA1 does not paint the inactive X to localize XIST RNA but may contribute to broad changes in cancer that impact XIST and Xi heterochromatin.J Cell Biochem. 2007 Mar 1;100(4):835-50. doi: 10.1002/jcb.21188. J Cell Biochem. 2007. PMID: 17146760
-
Replication of centromeric heterochromatin in mouse fibroblasts takes place in early, middle, and late S phase.Histochem Cell Biol. 2006 Jan;125(1-2):91-102. doi: 10.1007/s00418-005-0063-3. Epub 2005 Oct 18. Histochem Cell Biol. 2006. PMID: 16231189
-
Pericentric heterochromatin: dynamic organization during early development in mammals.Differentiation. 2008 Jan;76(1):15-23. doi: 10.1111/j.1432-0436.2007.00220.x. Epub 2007 Sep 6. Differentiation. 2008. PMID: 17825083 Review.
-
Who Needs This Junk, or Genomic Dark Matter.Biochemistry (Mosc). 2018 Apr;83(4):450-466. doi: 10.1134/S0006297918040156. Biochemistry (Mosc). 2018. PMID: 29626931 Review.
Cited by
-
TIAR marks nuclear G2/M transition granules and restricts CDK1 activity under replication stress.EMBO Rep. 2019 Jan;20(1):e46224. doi: 10.15252/embr.201846224. Epub 2018 Dec 11. EMBO Rep. 2019. PMID: 30538118 Free PMC article.
-
Human Wrnip1 is localized in replication factories in a ubiquitin-binding zinc finger-dependent manner.J Biol Chem. 2008 Dec 12;283(50):35173-85. doi: 10.1074/jbc.M803219200. Epub 2008 Oct 7. J Biol Chem. 2008. PMID: 18842586 Free PMC article.
-
Bi-phasic expression of Heterochromatin Protein 1 (HP1) during breast cancer progression: Potential roles of HP1 and chromatin structure in tumorigenesis.J Nat Sci. 2015;1(7):e127. J Nat Sci. 2015. PMID: 26082944 Free PMC article.
-
Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):285-90. doi: 10.1073/pnas.1309085110. Epub 2013 Dec 17. Proc Natl Acad Sci U S A. 2014. PMID: 24347639 Free PMC article.
-
BRCA1 prevents R-loop-associated centromeric instability.Cell Death Dis. 2021 Oct 1;12(10):896. doi: 10.1038/s41419-021-04189-3. Cell Death Dis. 2021. PMID: 34599155 Free PMC article.
References
-
- Bravo, R. 1986. Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp. Cell Res. 163:287–293. - PubMed
-
- Cantor, S.B., D.W. Bell, S. Ganesan, E.M. Kass, R. Drapkin, S. Grossman, D.C. Wahrer, D.C. Sgroi, W.S. Lane, D.A. Haber, and D.M. Livingston. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 105:149–160. - PubMed
-
- Chadwick, B.P., and T.F. Lane. 2005. BRCA1 associates with the inactive X chromosome in late S-phase, coupled with transient H2AX phosphorylation. Chromosoma. 114:432–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

