Influenza virus pleiomorphy characterized by cryoelectron tomography
- PMID: 17146053
- PMCID: PMC1748186
- DOI: 10.1073/pnas.0607614103
Influenza virus pleiomorphy characterized by cryoelectron tomography
Abstract
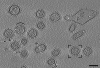
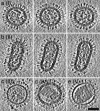
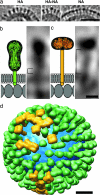
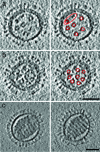
Influenza virus remains a global health threat, with millions of infections annually and the impending threat that a strain of avian influenza may develop into a human pandemic. Despite its importance as a pathogen, little is known about the virus structure, in part because of its intrinsic structural variability (pleiomorphy): the primary distinction is between spherical and elongated particles, but both vary in size. Pleiomorphy has thwarted structural analysis by image reconstruction of electron micrographs based on averaging many identical particles. In this study, we used cryoelectron tomography to visualize the 3D structures of 110 individual virions of the X-31 (H3N2) strain of influenza A. The tomograms distinguish two kinds of glycoprotein spikes [hemagglutinin (HA) and neuraminidase (NA)] in the viral envelope, resolve the matrix protein layer lining the envelope, and depict internal configurations of ribonucleoprotein (RNP) complexes. They also reveal the stems that link the glycoprotein ectodomains to the membrane and interactions among the glycoproteins, the matrix, and the RNPs that presumably control the budding of nascent virions from host cells. Five classes of virions, four spherical and one elongated, are distinguished by features of their matrix layer and RNP organization. Some virions have substantial gaps in their matrix layer ("molecular fontanels"), and others appear to lack a matrix layer entirely, suggesting the existence of an alternative budding pathway in which matrix protein is minimally involved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lamb RA, Krug RM. In: Fields Virology. Knipe DM, Howley PM, editors. Vol 1. New York: Lippincott Williams and Wilkins; 2001. pp. 1487–1579.
-
- Wilson IA, Skehel JJ, Wiley DC. Nature. 1981;289:366–373. - PubMed
-
- Varghese JN, Laver WG, Colman PM. Nature. 1983;303:35–40. - PubMed
-
- Harris A, Forouhar F, Qiu S, Sha B, Luo M. Virology. 2001;289:34–44. - PubMed
-
- Schulze IT. Virology. 1972;47:181–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

