Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells
- PMID: 17147513
- PMCID: PMC1828881
- DOI: 10.1042/BJ20061257
Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells
Abstract
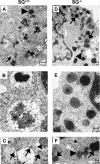
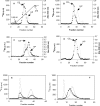
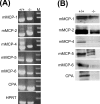
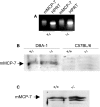
SG (serglycin) PGs (proteoglycans) are strongly implicated in the assembly of MC (mast cell) granules. However, this notion has mainly been on the basis of studies of MCs of the connective tissue subtype, whereas the role of SG PG in mucosal MCs has not been explored. In the present study, we have addressed the latter issue by using mice with an inactivated SG gene. Bone marrow cells were differentiated in vitro into the mucosal MC phenotype, expressing the markers mMCP (mouse MC protease) -1 and -2. Biosynthetic labelling experiments performed on these cells revealed an approximately 80% reduction of 35SO4(2-) incorporation into PGs recovered from SG-/- cells as compared with SG+/+ counterparts, indicating that SG is the dominating cell-associated PG of mucosal MCs. Moreover, the absence of SG led to defective metachromatic staining of mucosal MCs, both in vivo and in the in vitro-derived mucosal MCs. Ultrastructural analysis showed that granules were present in similar numbers in SG+/+ and SG-/- cells, but that their morphology was markedly affected by the absence of SG, e.g. with electron-dense core formation only seen in SG+/+ granules. Analysis of the MC-specific proteases showed that mMCP-1 and mMCP-7 were completely independent of SG for storage, whereas mMCP-2 showed a partial dependence. In contrast, mMCP-4 and -6, and carboxypeptidase A were strongly dependent on SG for storage. Together, our data indicate that SG PG is of crucial importance for assembly of mature mucosal MC granules, but that the specific dependence on SG for storage varies between individual granule constituents.
Figures


References
-
- Nawa Y., Kiyota M., Korenaga M., Kotani M. Defective protective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol. 1985;7:429–438. - PubMed
-
- Malaviya R., Ikeda T., Ross E., Abraham S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. - PubMed
-
- Echtenacher B., Mannel D. N., Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

