Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade
- PMID: 17147517
- PMCID: PMC1828883
- DOI: 10.1042/BJ20061520
Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade
Abstract
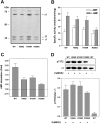
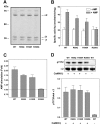
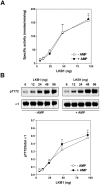
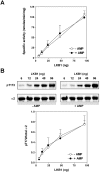
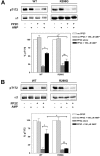
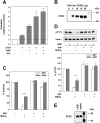
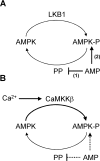
AMPK (AMP-activated protein kinase) is activated allosterically by AMP and by phosphorylation of Thr172 within the catalytic alpha subunit. Here we show that mutations in the regulatory gamma subunit reduce allosteric activation of the kinase by AMP. In addition to its allosteric effect, AMP significantly reduces the dephosphorylation of Thr172 by PP (protein phosphatase)2Calpha. Moreover, a mutation in the gamma subunit almost completely abolishes the inhibitory effect of AMP on dephosphorylation. We were unable to detect any effect of AMP on Thr172 phosphorylation by either LKB1 or CaMKKbeta (Ca2+/calmodulin-dependent protein kinase kinase beta) using recombinant preparations of the proteins. However, using partially purified AMPK from rat liver, there was an apparent AMP-stimulation of Thr172 phosphorylation by LKB1, but this was blocked by the addition of NaF, a PP inhibitor. Western blotting of partially purified rat liver AMPK and LKB1 revealed the presence of PP2Calpha in the preparations. We suggest that previous studies reporting that AMP promotes phosphorylation of Thr172 were misinterpreted. A plausible explanation for this effect of AMP is inhibition of dephosphorylation by PP2Calpha, present in the preparations of the kinases used in the earlier studies. Taken together, our results demonstrate that AMP activates AMPK via two mechanisms: by direct allosteric activation and by protecting Thr172 from dephosphorylation. On the basis of our new findings, we propose a simple model for the regulation of AMPK in mammalian cells by LKB1 and CaMKKbeta. This model accounts for activation of AMPK by two distinct signals: a Ca2+-dependent pathway, mediated by CaMKKbeta and an AMP-dependent pathway, mediated by LKB1.
Figures

References
-
- Carling D. The AMP-activated protein kinase cascade: a unifying system for energy control. Trends Biochem. Sci. 2004;29:18–24. - PubMed
-
- Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. - PubMed
-
- Hardie D. G., Carling D., Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998;67:821–855. - PubMed
-
- Sutherland C. M., Hawley S. A., McCartney R. R., Leech A., Stark M. J. R., Schmidt M. C., Hardie D. G. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr. Biol. 2003;13:1299–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

