Characterization of a beta-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry
- PMID: 17147821
- PMCID: PMC1712339
- DOI: 10.1186/1471-2091-7-23
Characterization of a beta-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry
Abstract
Background: In the barley malting process, partial hydrolysis of beta-glucans begins with seed germination. However, the endogenous 1,3-1,4-beta-glucanases are heat inactivated, and the remaining high molecular weight beta-glucans may cause severe problems such as increased brewer mash viscosity and turbidity. Increased viscosity impairs pumping and filtration, resulting in lower efficiency, reduced yields of extracts, and lower filtration rates, as well as the appearance of gelatinous precipitates in the finished beer. Therefore, the use of exogenous beta-glucanases to reduce the beta-glucans already present in the malt barley is highly desirable.
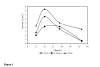
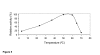
Results: The zygomycete microfungus Rhizopus microsporus var. microsporus secreted substantial amounts of beta-glucanase in liquid culture medium containing 0.5% chitin. An active protein was isolated by gel filtration and ion exchange chromatographies of the beta-glucanase activity-containing culture supernatant. This isolated protein hydrolyzed 1,3-1,4-beta-glucan (barley beta-glucan), but showed only residual activity against 1,3-beta-glucan (laminarin), or no activity at all against 1,4-beta-glucan (cellulose), indicating that the R. microsporus var. microsporus enzyme is a member of the EC 3.2.1.73 category. The purified protein had a molecular mass of 33.7 kDa, as determined by mass spectrometry. The optimal pH and temperature for hydrolysis of 1,3-1,4-beta-glucan were in the ranges of 4-5, and 50-60 degrees C, respectively. The Km and Vmax values for hydrolysis of beta-glucan at pH 5.0 and 50 degrees C were 22.39 mg.mL-1 and 16.46 mg.min-1, respectively. The purified enzyme was highly sensitive to Cu+2, but showed less or no sensitivity to other divalent ions, and was able to reduce both the viscosity and the filtration time of a sample of brewer mash. In comparison to the values determined for the mash treated with two commercial glucanases, the relative viscosity value for the mash treated with the 1,3-1,4-beta-glucanase produced by R. microsporus var. microsporus. was determined to be consistently lower.
Conclusion: The zygomycete microfungus R. microsporus var. microsporus produced a 1,3-1,4-beta-D-glucan 4-glucanhydrolase (EC 3.2.1.73) which is able to hydrolyze beta-D-glucan that contains both the 1,3- and 1,4-bonds (barley beta-glucans). Its molecular mass was 33.7 kDa. Maximum activity was detected at pH values in the range of 4-5, and temperatures in the range of 50-60 degrees C. The enzyme was able to reduce both the viscosity of the brewer mash and the filtration time, indicating its potential value for the brewing industry.
Figures





Similar articles
-
Enhanced hydrolytic efficiency of an engineered CBM11-glucanase enzyme chimera against barley β-d-glucan extracts.Food Chem. 2021 Dec 15;365:130460. doi: 10.1016/j.foodchem.2021.130460. Epub 2021 Jun 25. Food Chem. 2021. PMID: 34237573
-
Comparison of wild-type and UV-mutant beta-glucanase-producing strains of Talaromyces emersonii with potential in brewing applications.J Ind Microbiol Biotechnol. 2005 Apr;32(4):125-34. doi: 10.1007/s10295-005-0207-4. Epub 2005 Apr 22. J Ind Microbiol Biotechnol. 2005. PMID: 15856354
-
In vivo modeling of beta-glucan degradation in contrasting barley (Hordeum vulgare L.) genotypes.J Agric Food Chem. 2007 Apr 18;55(8):3158-66. doi: 10.1021/jf0636768. Epub 2007 Mar 24. J Agric Food Chem. 2007. PMID: 17381125
-
Fungal β-1,3-1,4-glucanases: production, proprieties and biotechnological applications.J Sci Food Agric. 2019 Apr;99(6):2657-2664. doi: 10.1002/jsfa.9491. Epub 2019 Jan 4. J Sci Food Agric. 2019. PMID: 30430579 Review.
-
Polysaccharide hydrolases in germinated barley and their role in the depolymerization of plant and fungal cell walls.Int J Biol Macromol. 1997 Aug;21(1-2):67-72. doi: 10.1016/s0141-8130(97)00043-3. Int J Biol Macromol. 1997. PMID: 9283018 Review.
Cited by
-
Purification, characterization, immobilization and applications of an enzybiotic β-1,3-1,4-glucanase produced from halotolerant marine Halomonas meridiana ES021.World J Microbiol Biotechnol. 2023 Feb 6;39(4):89. doi: 10.1007/s11274-023-03527-1. World J Microbiol Biotechnol. 2023. PMID: 36740637 Free PMC article.
-
Whole-genome de novo sequencing, combined with RNA-Seq analysis, reveals unique genome and physiological features of the amylolytic yeast Saccharomycopsis fibuligera and its interspecies hybrid.Biotechnol Biofuels. 2016 Nov 11;9:246. doi: 10.1186/s13068-016-0653-4. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27872659 Free PMC article.
-
Crystallization and preliminary X-ray analysis of a 1,3-1,4-beta-glucanase from Paecilomyces thermophila.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Aug 1;64(Pt 8):754-6. doi: 10.1107/S1744309108021064. Epub 2008 Jul 31. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008. PMID: 18678950 Free PMC article.
-
Mitochondrial genomic characteristics and phylogenetic analysis of a brewing fungus, Rhizopus microsporus Tiegh. 1875 (Mucorales: Rhizopodaceae).Mitochondrial DNA B Resour. 2024 May 20;9(5):657-662. doi: 10.1080/23802359.2024.2356133. eCollection 2024. Mitochondrial DNA B Resour. 2024. PMID: 38774188 Free PMC article.
-
Purification and characterization of a novel alkaline β-1,3-1,4-glucanase (lichenase) from thermophilic fungus Malbranchea cinnamomea.J Ind Microbiol Biotechnol. 2014 Oct;41(10):1487-95. doi: 10.1007/s10295-014-1494-4. Epub 2014 Aug 12. J Ind Microbiol Biotechnol. 2014. PMID: 25113013
References
-
- Stone BA, Clarke AE. Chemistry and Biology of 1,3-β-Glucans. Bundoora: La Trobe University Press;; 1992.
-
- Planas A. Bacterial 1,3-1,4-β-glucanases: structure, function and protein engineering. Biochimica et Biophysica Acta. 2000;1543:361–382. - PubMed
-
- Bamforth CW. β-Glucan and β-glucanases in malting and brewing: practical aspects. Brewers Digest. 1994;69:12–16.
-
- Fincher GB, Stone BA. Advances in cereal science and technology. Vol. 7. American Association of Cereal Chemists. St. Paul; 1986.
-
- Godfrey T, West S. Industrial enzymology. New York : Stockton Press; 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

