Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells
- PMID: 17148585
- PMCID: PMC1852212
- DOI: 10.1182/blood-2006-07-037754
Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells
Abstract
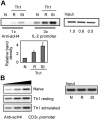
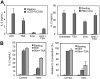
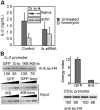
In T cells anergy may be evoked by an unbalanced stimulation of the T-cell receptor in the absence of costimulation. Anergic T cells are unresponsive to new antigen receptor engagement and do not produce interleukin 2. We present evidence that anergizing stimuli induce changes in histone acetylation, which mediates transcriptional repression of interleukin 2 expression. In response to calcium signaling, anergic T cells up-regulate the expression of Ikaros, a zinc finger transcription factor essential for lymphoid lineage determination. Ikaros binds to the interleukin 2 promoter where it induces histone deacetylation. Confirming the role of Ikaros in the induction of T-cell anergy, cells with reduced Ikaros activity show defective inactivation in response to an anergizing stimulus. We propose a model in which tolerizing stimuli induce epigenetic changes on the interleukin 2 locus that are responsible for the stable inhibition of the expression of this cytokine in anergic T cells.
Figures



References
-
- Abbas AK. The control of T cell activation vs tolerance. Autoimmun Rev. 2003;2:115–118. - PubMed
-
- Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. - PubMed
-
- Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. - PubMed
-
- Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

