Synapse-specific regulation of AMPA receptor function by PSD-95
- PMID: 17148601
- PMCID: PMC1748260
- DOI: 10.1073/pnas.0608492103
Synapse-specific regulation of AMPA receptor function by PSD-95
Abstract
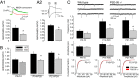
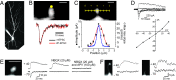
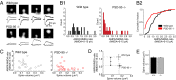
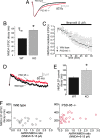
PSD-95 is a major protein found in virtually all mature excitatory glutamatergic synapses in the brain. Here, we have addressed the role of PSD-95 in controlling glutamatergic synapse function by generating and characterizing a PSD-95 KO mouse. We found that the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)subtype of glutamate receptor (AMPAR)-mediated synaptic transmission was reduced in these mice. Two-photon (2P) uncaging of MNI-glutamate onto individual spines suggested that the decrease in AMPAR function in the PSD-95 KO mouse stems from an increase in the proportion of "silent" synapses i.e., synapses containing N-methyl-d-aspartate (NMDA) receptors (NMDARs) but no AMPARs. Unexpectedly, the silent synapses in the KO mouse were located onto morphologically mature spines. We also observed that a significant population of synapses appeared unaffected by PSD-95 gene deletion, suggesting that the functional role of PSD-95 displays synapse-specificity. In addition, we report that the decay of NMDAR-mediated current was slower in KO mice: The contribution of NR2B subunit containing receptors to the NMDAR-mediated synaptic current was greater in KO mice. The greater occurrence of silent synapses might be related to the greater magnitude of potentiation after long-term potentiation induction observed in these mice. Together, these results suggest a synapse-specific role for PSD-95 in controlling synaptic function that is independent of spine morphology.
Conflict of interest statement
Conflict of interest statement: Under a licensing agreement between Upstate Group, Inc. and The Johns Hopkins University, R.L.H. is entitled to a share of royalties received by the University on sales of products described in this article. R.L.H. is a paid consultant to Upstate Group, Inc. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict-of-interest policies.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

