Regions of IkappaBalpha that are critical for its inhibition of NF-kappaB.DNA interaction fold upon binding to NF-kappaB
- PMID: 17148610
- PMCID: PMC1748158
- DOI: 10.1073/pnas.0605794103
Regions of IkappaBalpha that are critical for its inhibition of NF-kappaB.DNA interaction fold upon binding to NF-kappaB
Abstract
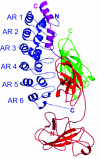
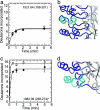
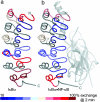
Nuclear factor kappaB (NF-kappaB) transcription factors regulate genes responsible for critical cellular processes. IkappaBalpha, -beta, and -epsilon bind to NF-kappaBs and inhibit their transcriptional activity. The NF-kappaB-binding domains of IkappaBs contain six ankyrin repeats (ARs), which adopt a beta-hairpin/alpha-helix/loop/alpha-helix/loop architecture. IkappaBalpha appears compactly folded in the IkappaBalpha.NF-kappaB crystal structure, but biophysical studies suggested that IkappaBalpha might be flexible even when bound to NF-kappaB. Amide H/(2)H exchange in free IkappaBalpha suggests that ARs 2-4 are compact, but ARs 1, 5, and 6 are conformationally flexible. Amide H/(2)H exchange is one of few techniques able to experimentally identify regions that fold upon binding. Comparison of amide H/(2)H exchange in free and NF-kappaB-bound IkappaBalpha reveals that the beta-hairpins in ARs 5 and 6 fold upon binding to NF-kappaB, but AR 1 remains highly solvent accessible. These regions are implicated in various aspects of NF-kappaB regulation, such as controlling degradation of IkappaBalpha, enabling high-affinity interaction with different NF-kappaB dimers, and preventing NF-kappaB from binding to its target DNA. Thus, IkappaBalpha conformational flexibility and regions of IkappaBalpha folding upon binding to NF-kappaB are important attributes for its regulation of NF-kappaB transcriptional activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


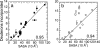
Similar articles
-
Pre-folding IkappaBalpha alters control of NF-kappaB signaling.J Mol Biol. 2008 Jun 27;380(1):67-82. doi: 10.1016/j.jmb.2008.02.053. Epub 2008 Mar 4. J Mol Biol. 2008. PMID: 18511071 Free PMC article.
-
Detection of a ternary complex of NF-kappaB and IkappaBalpha with DNA provides insights into how IkappaBalpha removes NF-kappaB from transcription sites.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1367-72. doi: 10.1073/pnas.1014323108. Epub 2011 Jan 10. Proc Natl Acad Sci U S A. 2011. PMID: 21220295 Free PMC article.
-
Solvent exposed non-contacting amino acids play a critical role in NF-kappaB/IkappaBalpha complex formation.J Mol Biol. 2002 Dec 6;324(4):587-97. doi: 10.1016/s0022-2836(02)01149-x. J Mol Biol. 2002. PMID: 12460563
-
Molecular mechanisms of system control of NF-kappaB signaling by IkappaBalpha.Biochemistry. 2010 Mar 2;49(8):1560-7. doi: 10.1021/bi901948j. Biochemistry. 2010. PMID: 20055496 Free PMC article. Review.
-
Regulation of IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages.Mediators Inflamm. 2010;2010:823821. doi: 10.1155/2010/823821. Epub 2010 Apr 12. Mediators Inflamm. 2010. PMID: 20396415 Free PMC article. Review.
Cited by
-
Role of disorder in IκB-NFκB interaction.IUBMB Life. 2012 Jun;64(6):499-505. doi: 10.1002/iub.1044. Epub 2012 May 9. IUBMB Life. 2012. PMID: 22573609 Free PMC article. Review.
-
The IkappaBalpha/NF-kappaB complex has two hot spots, one at either end of the interface.Protein Sci. 2008 Dec;17(12):2051-8. doi: 10.1110/ps.037481.108. Epub 2008 Sep 29. Protein Sci. 2008. PMID: 18824506 Free PMC article.
-
Structural insights into fibrinogen dynamics using amide hydrogen/deuterium exchange mass spectrometry.Biochemistry. 2013 Aug 13;52(32):5491-502. doi: 10.1021/bi4007995. Epub 2013 Aug 2. Biochemistry. 2013. PMID: 23875785 Free PMC article.
-
Structural modeling of the N-terminal signal-receiving domain of IκBα.Front Mol Biosci. 2015 Jun 23;2:32. doi: 10.3389/fmolb.2015.00032. eCollection 2015. Front Mol Biosci. 2015. PMID: 26157801 Free PMC article.
-
Observation of solvent penetration during cold denaturation of E. coli phosphofructokinase-2.Biophys J. 2013 May 21;104(10):2254-63. doi: 10.1016/j.bpj.2013.04.024. Biophys J. 2013. PMID: 23708365 Free PMC article.
References
-
- Pahl HL. Oncogene. 1999;18:6853–6866. - PubMed
-
- Ghosh S, May MJ, Kopp EB. Annu Rev Immunol. 1998;16:225–260. - PubMed
-
- Li X, Stark GR. Exp Hematol. 2002;30:285–296. - PubMed
-
- Bonizzi G, Karin M. Trends Immunol. 2004;25:280–288. - PubMed
-
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. J Mol Med. 2004;82:434–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

