Regions of IkappaBalpha that are critical for its inhibition of NF-kappaB.DNA interaction fold upon binding to NF-kappaB
- PMID: 17148610
- PMCID: PMC1748158
- DOI: 10.1073/pnas.0605794103
Regions of IkappaBalpha that are critical for its inhibition of NF-kappaB.DNA interaction fold upon binding to NF-kappaB
Abstract
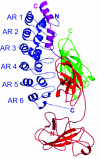
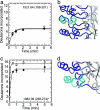
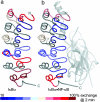
Nuclear factor kappaB (NF-kappaB) transcription factors regulate genes responsible for critical cellular processes. IkappaBalpha, -beta, and -epsilon bind to NF-kappaBs and inhibit their transcriptional activity. The NF-kappaB-binding domains of IkappaBs contain six ankyrin repeats (ARs), which adopt a beta-hairpin/alpha-helix/loop/alpha-helix/loop architecture. IkappaBalpha appears compactly folded in the IkappaBalpha.NF-kappaB crystal structure, but biophysical studies suggested that IkappaBalpha might be flexible even when bound to NF-kappaB. Amide H/(2)H exchange in free IkappaBalpha suggests that ARs 2-4 are compact, but ARs 1, 5, and 6 are conformationally flexible. Amide H/(2)H exchange is one of few techniques able to experimentally identify regions that fold upon binding. Comparison of amide H/(2)H exchange in free and NF-kappaB-bound IkappaBalpha reveals that the beta-hairpins in ARs 5 and 6 fold upon binding to NF-kappaB, but AR 1 remains highly solvent accessible. These regions are implicated in various aspects of NF-kappaB regulation, such as controlling degradation of IkappaBalpha, enabling high-affinity interaction with different NF-kappaB dimers, and preventing NF-kappaB from binding to its target DNA. Thus, IkappaBalpha conformational flexibility and regions of IkappaBalpha folding upon binding to NF-kappaB are important attributes for its regulation of NF-kappaB transcriptional activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


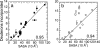
References
-
- Pahl HL. Oncogene. 1999;18:6853–6866. - PubMed
-
- Ghosh S, May MJ, Kopp EB. Annu Rev Immunol. 1998;16:225–260. - PubMed
-
- Li X, Stark GR. Exp Hematol. 2002;30:285–296. - PubMed
-
- Bonizzi G, Karin M. Trends Immunol. 2004;25:280–288. - PubMed
-
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. J Mol Med. 2004;82:434–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

