The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches
- PMID: 17148611
- PMCID: PMC1748200
- DOI: 10.1073/pnas.0605275103
The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches
Abstract
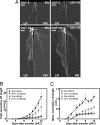
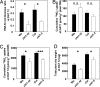
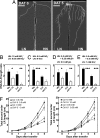
Localized proliferation of lateral roots in NO(3)(-)-rich patches is a striking example of the nutrient-induced plasticity of root development. In Arabidopsis, NO(3)(-) stimulation of lateral root elongation is apparently under the control of a NO(3)(-)-signaling pathway involving the ANR1 transcription factor. ANR1 is thought to transduce the NO(3)(-) signal internally, but the upstream NO(3)(-) sensing system is unknown. Here, we show that mutants of the NRT1.1 nitrate transporter display a strongly decreased root colonization of NO(3)(-)-rich patches, resulting from reduced lateral root elongation. This phenotype is not due to lower specific NO(3)(-) uptake activity in the mutants and is not suppressed when the NO(3)(-)-rich patch is supplemented with an alternative N source but is associated with dramatically decreased ANR1 expression. These results show that NRT1.1 promotes localized root proliferation independently of any nutritional effect and indicate a role in the ANR1-dependent NO(3)(-) signaling pathway, either as a NO(3)(-) sensor or as a facilitator of NO(3)(-) influx into NO(3)(-)-sensing cells. Consistent with this model, the NRT1.1 and ANR1 promoters both directed reporter gene expression in root primordia and root tips. The inability of NRT1.1-deficient mutants to promote increased lateral root proliferation in the NO(3)(-)-rich zone impairs the efficient acquisition of NO(3)(-) and leads to slower plant growth. We conclude that NRT1.1, which is localized at the forefront of soil exploration by the roots, is a key component of the NO(3)(-)-sensing system that enables the plant to detect and exploit NO(3)(-)-rich soil patches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability.Plant Cell Environ. 2014 Jan;37(1):162-74. doi: 10.1111/pce.12143. Epub 2013 Jun 25. Plant Cell Environ. 2014. PMID: 23731054
-
Nitrate regulation of AFB3 and NAC4 gene expression in Arabidopsis roots depends on NRT1.1 nitrate transport function.Plant Signal Behav. 2014;9(3):e28501. doi: 10.4161/psb.28501. Epub 2014 Jan 1. Plant Signal Behav. 2014. PMID: 24642706 Free PMC article.
-
Overexpressing the ANR1 MADS-box gene in transgenic plants provides new insights into its role in the nitrate regulation of root development.Plant Cell Physiol. 2012 Jun;53(6):1003-16. doi: 10.1093/pcp/pcs050. Epub 2012 Apr 19. Plant Cell Physiol. 2012. PMID: 22523192
-
Nitrate Signaling, Functions, and Regulation of Root System Architecture: Insights from Arabidopsis thaliana.Genes (Basel). 2020 Jun 9;11(6):633. doi: 10.3390/genes11060633. Genes (Basel). 2020. PMID: 32526869 Free PMC article. Review.
-
Nitrate transceptor(s) in plants.J Exp Bot. 2011 Apr;62(7):2299-308. doi: 10.1093/jxb/erq419. Epub 2011 Jan 14. J Exp Bot. 2011. PMID: 21239382 Review.
Cited by
-
Improved Plant Nitrate Status Involves in Flowering Induction by Extended Photoperiod.Front Plant Sci. 2021 Feb 12;12:629857. doi: 10.3389/fpls.2021.629857. eCollection 2021. Front Plant Sci. 2021. PMID: 33643357 Free PMC article.
-
NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis.Nat Commun. 2016 Oct 12;7:13179. doi: 10.1038/ncomms13179. Nat Commun. 2016. PMID: 27731416 Free PMC article.
-
LjNRT2.3 plays a hierarchical role in the control of high affinity transport system for root nitrate acquisition in Lotus japonicus.Front Plant Sci. 2022 Nov 10;13:1042513. doi: 10.3389/fpls.2022.1042513. eCollection 2022. Front Plant Sci. 2022. PMID: 36438153 Free PMC article.
-
Nitrate Controls Root Development through Posttranscriptional Regulation of the NRT1.1/NPF6.3 Transporter/Sensor.Plant Physiol. 2016 Oct;172(2):1237-1248. doi: 10.1104/pp.16.01047. Epub 2016 Aug 19. Plant Physiol. 2016. PMID: 27543115 Free PMC article.
-
The MADS transcription factor CmANR1 positively modulates root system development by directly regulating CmPIN2 in chrysanthemum.Hortic Res. 2018 Oct 1;5:52. doi: 10.1038/s41438-018-0061-y. eCollection 2018. Hortic Res. 2018. PMID: 30302256 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

