Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas' disease
- PMID: 17148660
- PMCID: PMC1762476
- DOI: 10.2353/ajpath.2006.060475
Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas' disease
Abstract
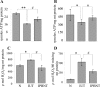
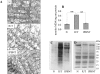
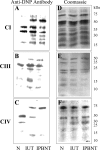
In this study, we investigated the mechanism(s) of mitochondrial functional decline in acute Chagas' disease. Our data show a substantial decline in respiratory complex activities (39 to 58%) and ATP (38%) content in Trypanosoma cruzi-infected murine hearts compared with normal controls. These metabolic alterations were associated with an approximately fivefold increase in mitochondrial reactive oxygen species production rate, substantial oxidative insult of mitochondrial membranes and respiratory complex subunits, and >60% inhibition of mtDNA-encoded transcripts for respiratory complex subunits in infected myocardium. The antioxidant phenyl-alpha-tert-butyl nitrone (PBN) arrested the oxidative damage-mediated loss in mitochondrial membrane integrity, preserved redox potential-coupled mitochondrial gene expression, and improved respiratory complex activities (47 to 95% increase) and cardiac ATP level (>or=40% increase) in infected myocardium. Importantly, PBN resulted twofold decline in mitochondrial reactive oxygen species production rate in infected myocardium. Taken together, our data demonstrate the pathological significance of oxidative stress in metabolic decay and energy homeostasis in acute chagasic myocarditis and further suggest that oxidative injuries affecting mitochondrial integrity-dependent expression and activity of the respiratory complexes initiate a feedback cycle of electron transport chain inefficiency, increased reactive oxygen species production, and energy homeostasis in acute chagasic hearts. PBN and other mitochondria-targeted antioxidants may be useful in altering mitochondrial decay and oxidative pathology in Chagas' disease.
Figures
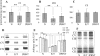


Similar articles
-
Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi-infected mice.Antioxid Redox Signal. 2010 Jan;12(1):27-37. doi: 10.1089/ars.2008.2418. Antioxid Redox Signal. 2010. PMID: 19624257 Free PMC article.
-
Mitochondrial generation of reactive oxygen species is enhanced at the Q(o) site of the complex III in the myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant.J Bioenerg Biomembr. 2008 Dec;40(6):587-98. doi: 10.1007/s10863-008-9184-4. Epub 2008 Nov 14. J Bioenerg Biomembr. 2008. PMID: 19009337 Free PMC article.
-
Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic Rats.J Am Coll Cardiol. 2010 Jun 1;55(22):2499-508. doi: 10.1016/j.jacc.2010.02.030. J Am Coll Cardiol. 2010. PMID: 20510218 Free PMC article.
-
Mitochondrial oxidative stress and dysfunction in myocardial remodelling.Cardiovasc Res. 2009 Feb 15;81(3):449-56. doi: 10.1093/cvr/cvn280. Epub 2008 Oct 14. Cardiovasc Res. 2009. PMID: 18854381 Review.
-
An overview of chagasic cardiomyopathy: pathogenic importance of oxidative stress.An Acad Bras Cienc. 2005 Dec;77(4):695-715. doi: 10.1590/s0001-37652005000400009. Epub 2005 Nov 29. An Acad Bras Cienc. 2005. PMID: 16341444 Review.
Cited by
-
Cardiac-oxidized antigens are targets of immune recognition by antibodies and potential molecular determinants in chagas disease pathogenesis.PLoS One. 2012;7(1):e28449. doi: 10.1371/journal.pone.0028449. Epub 2012 Jan 4. PLoS One. 2012. PMID: 22238578 Free PMC article.
-
Immunity and vaccine development efforts against Trypanosoma cruzi.Acta Trop. 2019 Dec;200:105168. doi: 10.1016/j.actatropica.2019.105168. Epub 2019 Sep 9. Acta Trop. 2019. PMID: 31513763 Free PMC article. Review.
-
Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi-infected mice.Antioxid Redox Signal. 2010 Jan;12(1):27-37. doi: 10.1089/ars.2008.2418. Antioxid Redox Signal. 2010. PMID: 19624257 Free PMC article.
-
Mitochondrial generation of reactive oxygen species is enhanced at the Q(o) site of the complex III in the myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant.J Bioenerg Biomembr. 2008 Dec;40(6):587-98. doi: 10.1007/s10863-008-9184-4. Epub 2008 Nov 14. J Bioenerg Biomembr. 2008. PMID: 19009337 Free PMC article.
-
Developments in the management of Chagas cardiomyopathy.Expert Rev Cardiovasc Ther. 2015 Dec;13(12):1393-409. doi: 10.1586/14779072.2015.1103648. Epub 2015 Oct 23. Expert Rev Cardiovasc Ther. 2015. PMID: 26496376 Free PMC article. Review.
References
-
- World Health Organization: Control of Chagas Disease: WHO Technical Report Series 905. Second Report of the WHO Expert Committee. Geneva, Switzerland, 2002, pp 1–99
-
- Carrasco Guerra HA, Palacios-Pru E, Dagert de Scorza C, Molina C, Inglessis G, Mendoza RV. Clinical, histochemical, and ultrastructural correlation in septal endomyocardial biopsies from chronic chagasic patients: detection of early myocardial damage. Am Heart J. 1987;113:716–724. - PubMed
-
- Palacios-Pru E, Carrasco H, Scorza C, Espinoza R. Ultrastructural characteristics of different stages of human chagasic myocarditis. Am J Trop Med Hyg. 1989;41:29–40. - PubMed
-
- Parada H, Carrasco HA, Anez N, Fuenmayor C, Inglessis I. Cardiac involvement is a constant finding in acute Chagas’ disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997;60:49–54. - PubMed
-
- Garg N, Popov VL, Papaconstantinou J. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: implications in chagasic myocarditis development. Biochim Biophys Acta. 2003;1638:106–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

