Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells
- PMID: 17148667
- PMCID: PMC1762467
- DOI: 10.2353/ajpath.2006.060081
Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells
Abstract
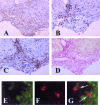
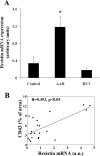
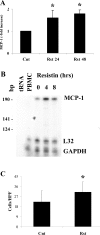
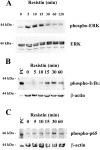
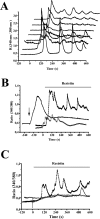
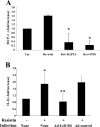
Obesity and insulin resistance accelerate the progression of fibrosis during chronic liver disease. Resistin antagonizes insulin action in rodents, but its role in humans is still controversial. The aims of this study were to investigate resistin expression in human liver and to evaluate whether resistin may affect the biology of activated human hepatic stellate cells (HSCs), key modulators of hepatic fibrogenesis. Resistin gene expression was low in normal human liver but was increased in conditions of severe fibrosis. Up-regulation of resistin during chronic liver damage was confirmed by immunohistochemistry. In a group of patients with alcoholic hepatitis, resistin expression correlated with inflammation and fibrosis, suggesting a possible action on HSCs. Exposure of cultured HSCs to recombinant resistin resulted in increased expression of the proinflammatory chemokines monocyte chemoattractant protein-1 and interleukin-8, through activation of nuclear factor (NF)-kappaB. Resistin induced a rapid increase in intracellular calcium concentration, mainly through calcium release from intracellular inositol triphosphate-sensitive pools. The intracellular calcium chelator BAPTA-AM blocked resistin-induced NF-kappaB activation and monocyte chemoattractant protein-1 expression. In conclusion, this study shows a role for resistin as an intrahepatic cytokine exerting proinflammatory actions in HSCs, via a Ca2+/NF-kappaB-dependent pathway and suggests involvement of this adipokine in the pathophysiology of liver fibrosis.
Figures
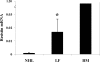

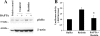
References
-
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. - PubMed
-
- Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. - PubMed
-
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

