Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals
- PMID: 17148678
- PMCID: PMC1762489
- DOI: 10.2353/ajpath.2006.060329
Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals
Abstract
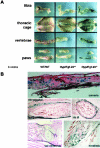
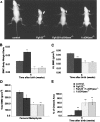
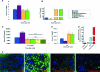
Fibroblast growth factor-23 (FGF-23) is one of the circulating phosphaturic factors associated with renal phosphate wasting. Fgf-23-/- animals show extremely high serum levels of phosphate and 1,25-dihydroxyvitamin D3, along with abnormal bone mineralization and soft tissue calcifications. To determine the role of vitamin D in mediating altered phosphate homeostasis and skeletogenesis in the Fgf-23-/- mice, we generated mice lacking both the Fgf-23 and 1alpha-hydroxylase genes (Fgf-23-/-/1alpha(OH)ase-/-). In the current study, we have identified the cellular source of Fgf-23 in adult mice. In addition, loss of vitamin D activities from Fgf-23-/- mice reverses the severe hyperphosphatemia to hypophosphatemia, attributable to increased urinary phosphate wasting in Fgf-23-/-/1alpha(OH)ase-/- mice, possibly as a consequence of decreased expression of NaPi2a. Ablation of vitamin D from Fgf-23-/- mice resulted in further reduction of total bone mineral content and bone mineral density and reversed ectopic calcification of skeleton and soft tissues, suggesting that abnormal mineral ion homeostasis and impaired skeletogenesis in Fgf-23-/- mice are mediated through enhanced vitamin D activities. In conclusion, using genetic manipulation studies, we have provided evidence for an in vivo inverse correlation between Fgf-23 and vitamin D activities and for the severe skeletal and soft tissue abnormalities of Fgf-23-/- mice being mediated through vitamin D.
Figures


References
-
- Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. Philadelphia: WB Saunders Co.,; Williams Textbook of Endocrinology. 1998:pp 1155–1210.
-
- White KE, Evans WE, O’Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, Grabowski M, Mettinger T, Strom TM. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat Genet. 2000;26:345–348. - PubMed
-
- Francis F, Henning S, Korn B, Reinhardt R, de Jong P, Poustka A, Lehrach H, Rowe PSN, Goulding JN, Summerfield T, Mountford R, Read AP, Popowska E, Pronicka E, Davies KE, O’Riordan JLH, Econs MJ, Nesbitt T, Drezner MK, Oudet C, Pannetier S, Hanauer A, Strom TM, Meindl A, Lorenz B, Cagnoli M, Mohnike KL, Murken J, Meitinger T. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HPY Consortium. Nat Genet. 1995;11:130–136. - PubMed
-
- Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

