An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction
- PMID: 17148684
- PMCID: PMC1762477
- DOI: 10.2353/ajpath.2006.060398
An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction
Abstract
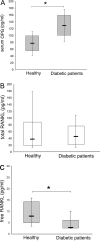
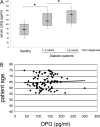
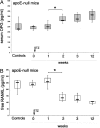
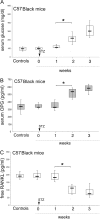
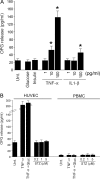
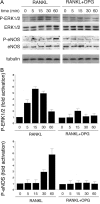
Serum osteoprotegerin (OPG) is significantly increased in diabetic patients, prompting expanded investigation of the correlation between OPG production/release and glycemic levels. Serum levels of OPG, but not of its cognate ligand receptor activator of nuclear factor-kappaB ligand (RANKL), were significantly increased in type 2 diabetes mellitus patients compared with healthy blood donors. Serum OPG was also significantly elevated in a subgroup of recently diagnosed diabetic patients (within 2 years). The relationship between serum OPG and diabetes mellitus onset was next investigated in apoE-null and littermate mice. Serum OPG increased early after diabetes induction in both mouse strains and showed a positive correlation with blood glucose levels and an inverse correlation with the levels of free (OPG-unbound) RANKL. The in vitro addition of tumor necrosis factor-alpha to human vascular endothelial cells, but not human peripheral blood mononuclear cells, markedly enhanced OPG release in culture. In contrast, high glucose concentrations did not modulate OPG release when used alone or in association with tumor necrosis factor-alpha. Moreover, the ability of soluble RANKL to activate the extracellular signal-regulated kinase/mitogen-activated protein kinase and endothelial nitric-oxide synthase pathways in endothelial cells was neutralized by preincubation with recombinant OPG. Altogether, these findings suggest that increased OPG production represents an early event in the natural history of diabetes mellitus, possibly contributing to disease-associated endothelial cell dysfunction.
Figures


References
-
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. - PubMed
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96:3540–3545. - PMC - PubMed
-
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2418. - PMC - PubMed
-
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

