Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB
- PMID: 17148685
- PMCID: PMC1762469
- DOI: 10.2353/ajpath.2006.050706
Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB
Abstract
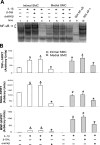
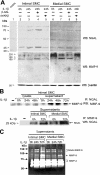
Neutrophil gelatinase-associated lipocalin (NGAL) has recently emerged as an important modulator of cell homeostasis. Elevated plasma NGAL levels, possibly because of activation of blood leukocytes, are associated with atherosclerosis. However, little is known about induction of NGAL expression in blood vessels. Using a rat carotid artery injury model, we found that NGAL was highly induced in the intima after angioplasty but was attenuated by adenovirus-mediated expression of a dominant-negative mutant of inhibitor of nuclear factor (NF)-kappaB kinase beta (dnIKKbeta). Expression of NGAL mRNA and protein was also up-regulated in an NF-kappaB-dependent manner in rat and human vascular smooth muscle cells (SMCs) in response to interleukin-1beta stimulation. Rat SMC-produced NGAL was present as mono- and homomeric forms in the cytosol and in a complex containing matrix metalloproteinase-9 (MMP-9) after secretion. In agreement with levels of NGAL, proteolytic activity of MMP-9 was markedly high in the intima of injured vessels and in the culture supernatant of activated intimal SMCs but was reduced in the vessels transduced with dnIKKbeta. The present study reveals a previously unrecognized vascular response to an-gioplastic injury, characterized by NF-kappaB-dependent expression of NGAL in vascular SMCs. Further-more, SMC-produced NGAL interacts with MMP-9, a mechanism by which NGAL may modulate MMP-9 proteolytic activity in the vascular repair process.
Figures




References
-
- Hraba-Renevey S, Turler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene. 1989;4:601–608. - PubMed
-
- Triebel S, Blaser J, Reinke H, Tschesche H. A 25 kDa alpha 2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–388. - PubMed
-
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. - PubMed
-
- Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem. 2001;268:1918–1928. - PubMed
-
- Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

