Serum antibody response to Human papillomavirus (HPV) infections detected by a novel ELISA technique based on denatured recombinant HPV16 L1, L2, E4, E6 and E7 proteins
- PMID: 17150135
- PMCID: PMC1660559
- DOI: 10.1186/1750-9378-1-6
Serum antibody response to Human papillomavirus (HPV) infections detected by a novel ELISA technique based on denatured recombinant HPV16 L1, L2, E4, E6 and E7 proteins
Abstract
Background: Human papillomaviruses (HPVs) are the primary etiological agents of cervical cancer and are also involved in the development of other tumours (skin, head and neck). Serological survey of the HPV infections is important to better elucidate their natural history and to disclose antigen determinants useful for vaccine development. At present, the analysis of the HPV-specific antibodies has not diagnostic value for the viral infections, and new approaches are needed to correlate the antibody response to the disease outcome. The aim of this study is to develop a novel ELISA, based on five denatured recombinant HPV16 proteins, to be used for detection HPV-specific antibodies.
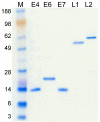
Methods: The HPV16 L1, L2, E4, E6 and E7 genes were cloned in a prokaryotic expression vector and expressed as histidine-tagged proteins. These proteins, in a denatured form, were used in ELISA as coating antigens. Human sera were collected from women with abnormal PAP smear enrolled during an ongoing multicenter HPV-PathogenISS study in Italy, assessing the HPV-related pathogenetic mechanisms of progression of cervical cancer precursor lesions. Negative human sera were collected from patients affected by other infectious agents. All the HPV-positive sera were also subjected to an avidity test to assess the binding strength in the antigen-antibody complexes.
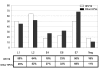
Results: Most of the sera showed a positive reactivity to the denatured HPV16 proteins: 82% of the sera from HPV16 infected women and 89% of the sera from women infected by other HPV genotypes recognised at least one of the HPV16 proteins. The percentages of samples showing reactivity to L1, L2 and E7 were similar, but only a few serum samples reacted to E6 and E4. Most sera bound the antigens with medium and high avidity index, suggesting specific antigen-antibody reactions.
Conclusion: This novel ELISA, based on multiple denatured HPV16 antigens, is able to detect antibodies in women infected by HPV16 and it is not genotype-specific, as it detects antibodies also in women infected by other genital HPVs. The assay is easy to perform and has low cost, making it suitable for monitoring the natural history of HPV infections as well as for detecting pre-existing HPV antibodies in women who receive VLP-based HPV vaccination.
Figures

Similar articles
-
Serological study in Tunisian cervical cancer patients.Pathol Biol (Paris). 2009 Jul;57(5):415-9. doi: 10.1016/j.patbio.2008.05.004. Epub 2008 Jun 30. Pathol Biol (Paris). 2009. PMID: 18586408
-
A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16.J Natl Cancer Inst. 1994 Apr 6;86(7):494-9. doi: 10.1093/jnci/86.7.494. J Natl Cancer Inst. 1994. PMID: 8133532 Free PMC article.
-
HPV-16-related proteins as the serologic markers in cervical neoplasia.Gynecol Oncol. 1998 Apr;69(1):47-55. doi: 10.1006/gyno.1998.4963. Gynecol Oncol. 1998. PMID: 9570998
-
Characterization of the humoral immune response to genital papillomaviruses.Mol Biol Med. 1990 Feb;7(1):59-72. Mol Biol Med. 1990. PMID: 2157937 Review.
-
Current Updates on Cancer-Causing Types of Human Papillomaviruses (HPVs) in East, Southeast, and South Asia.Cancers (Basel). 2021 May 30;13(11):2691. doi: 10.3390/cancers13112691. Cancers (Basel). 2021. PMID: 34070706 Free PMC article. Review.
Cited by
-
Purification and Characterization of Antibodies in Single-Chain Format against the E6 Oncoprotein of Human Papillomavirus Type 16.Biomed Res Int. 2018 May 20;2018:6583852. doi: 10.1155/2018/6583852. eCollection 2018. Biomed Res Int. 2018. PMID: 29888271 Free PMC article.
-
Clinical and epidemiological correlates of antibody response to human papillomaviruses (HPVs) as measured by a novel ELISA based on denatured recombinant HPV16 late (L) and early (E) antigens.Infect Agent Cancer. 2008 Jun 26;3:9. doi: 10.1186/1750-9378-3-9. Infect Agent Cancer. 2008. PMID: 18582363 Free PMC article.
-
Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer.Br J Cancer. 2011 Jun 7;104(12):1896-905. doi: 10.1038/bjc.2011.171. Br J Cancer. 2011. PMID: 21654689 Free PMC article.
-
HPV-E7 delivered by engineered exosomes elicits a protective CD8⁺ T cell-mediated immune response.Viruses. 2015 Mar 9;7(3):1079-99. doi: 10.3390/v7031079. Viruses. 2015. PMID: 25760140 Free PMC article.
-
Epitope Mapping and Computational Analysis of Anti-HPV16 E6 and E7 Antibodies in Single-Chain Format for Clinical Development as Antitumor Drugs.Cancers (Basel). 2020 Jul 6;12(7):1803. doi: 10.3390/cancers12071803. Cancers (Basel). 2020. PMID: 32640530 Free PMC article.
References
-
- Syrjänen K, Syrjänen S. Papillomavirus Infections in Human Pathology. Chichester: John Wiley & Sons Ltd;; 2000. pp. 1–615.
-
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157–1164. doi: 10.1158/1055-9965.EPI-04-0812. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

