Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo
- PMID: 17150307
- PMCID: PMC1944995
- DOI: 10.1016/j.neuroscience.2006.09.064
Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo
Abstract
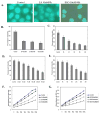
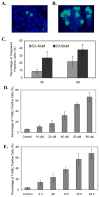
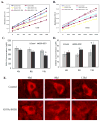
The mechanism of selective and age-dependent motor neuron degeneration in human amyotrophic lateral sclerosis (ALS) has not been defined and the role of glutathione (GSH) in association with motor neuron death remains largely unknown. A motor neuron-like cell culture system and a transgenic mouse model were used to study the effect of cellular GSH alteration on motor neuron cell death. Exposure of NSC34 motor neuron-like cells to ethacrynic acid (EA) or l-buthionine sulfoximine (BSO) dramatically reduced the cellular GSH levels, and was accompanied by increased production of reactive oxygen species (ROS) measured by the dichlorofluorescin (DCF) fluorescent oxidation assay. In addition, GSH depletion enhanced oxidative stress markers, AP-1 transcriptional activation, c-Jun, c-Fos and heme oxygenase-1 (HO-1) expression in NSC34 cells analyzed by a luciferase reporter, Western blotting and quantitative PCR assays respectively. Furthermore, depletion of GSH decreased mitochondrial function, facilitated apoptosis inducing factor (AIF) translocation, cytochrome c release, and caspase 3 activation, and consequently led to motor neuron-like cell apoptosis. In an ALS-like transgenic mouse model overexpressing mutant G93A-Cu, Zn-superoxide dismutase (SOD1) gene, we showed that the reduction of GSH in the spinal cord and motor neuron cells is correlated with AIF translocation, caspase 3 activation, and motor neuron degeneration during ALS-like disease onset and progression. Taken together, the in vitro and in vivo data presented in the current report demonstrated that decreased GSH promotes multiple apoptotic pathways contributing, at least partially, to motor neuron degeneration in ALS.
Figures







References
-
- Adams JD, Jr, Klaidman LK, Odunze IN, Shen HC, Miller CA. Alzheimer's and Parkinson's disease. Brain levels of glutathione, glutathione disulfide, and vitamin E. Mol Chem Neuropathol. 1991;14:213–226. - PubMed
-
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. - PubMed
-
- Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson's disease. Biochem Pharmacol. 2002;64:1037–1048. - PubMed
-
- Bishop A, Marquis JC, Cashman NR, Demple B. Adaptive resistance to nitric oxide in motor neurons. Free Radic Biol Med. 1999;26:978–986. - PubMed
-
- Bogdanov MB, Ramos LE, Xu Z, Beal MF. Elevated "hydroxyl radical" generation in vivo in an animal model of amyotrophic lateral sclerosis. J Neurochem. 1998;71:1321–1324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

