A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture
- PMID: 17151019
- PMCID: PMC2391258
- DOI: 10.1074/mcp.M600250-MCP200
A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture
Abstract
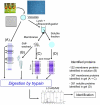
To better understand the mechanisms governing cellular traffic, storage of various metabolites, and their ultimate degradation, Arabidopsis thaliana vacuole proteomes were established. To this aim, a procedure was developed to prepare highly purified vacuoles from protoplasts isolated from Arabidopsis cell cultures using Ficoll density gradients. Based on the specific activity of the vacuolar marker alpha-mannosidase, the enrichment factor of the vacuoles was estimated at approximately 42-fold with an average yield of 2.1%. Absence of significant contamination by other cellular compartments was validated by Western blot using antibodies raised against specific markers of chloroplasts, mitochondria, plasma membrane, and endoplasmic reticulum. Based on these results, vacuole preparations showed the necessary degree of purity for proteomics study. Therefore, a proteomics approach was developed to identify the protein components present in both the membrane and soluble fractions of the Arabidopsis cell vacuoles. This approach includes the following: (i) a mild oxidation step leading to the transformation of cysteine residues into cysteic acid and methionine to methionine sulfoxide, (ii) an in-solution proteolytic digestion of very hydrophobic proteins, and (iii) a prefractionation of proteins by short migration by SDS-PAGE followed by analysis by liquid chromatography coupled to tandem mass spectrometry. This procedure allowed the identification of more than 650 proteins, two-thirds of which copurify with the membrane hydrophobic fraction and one-third of which copurifies with the soluble fraction. Among the 416 proteins identified from the membrane fraction, 195 were considered integral membrane proteins based on the presence of one or more predicted transmembrane domains, and 110 transporters and related proteins were identified (91 putative transporters and 19 proteins related to the V-ATPase pump). With regard to function, about 20% of the proteins identified were known previously to be associated with vacuolar activities. The proteins identified are involved in ion and metabolite transport (26%), stress response (9%), signal transduction (7%), and metabolism (6%) or have been described to be involved in typical vacuolar activities, such as protein and sugar hydrolysis. The subcellular localization of several putative vacuolar proteins was confirmed by transient expression of green fluorescent protein fusion constructs.
Figures



Similar articles
-
The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins.Plant Cell. 2004 Dec;16(12):3285-303. doi: 10.1105/tpc.104.027078. Epub 2004 Nov 11. Plant Cell. 2004. PMID: 15539469 Free PMC article.
-
A Proteomics Approach Highlights a Myriad of Transporters in the Arabidopsis thaliana Vacuolar Membrane.Plant Signal Behav. 2007 Sep;2(5):413-5. doi: 10.4161/psb.2.5.4415. Plant Signal Behav. 2007. PMID: 19704618 Free PMC article.
-
Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds.Plant Physiol. 2007 Sep;145(1):216-29. doi: 10.1104/pp.107.096917. Epub 2007 Jul 27. Plant Physiol. 2007. PMID: 17660356 Free PMC article.
-
The riddle of the plant vacuolar sorting receptors.Protoplasma. 2005 Dec;226(3-4):103-8. doi: 10.1007/s00709-005-0117-3. Epub 2005 Dec 12. Protoplasma. 2005. PMID: 16333569 Review.
-
The proteomics of plant cell membranes.J Exp Bot. 2007;58(1):103-12. doi: 10.1093/jxb/erj209. Epub 2006 Jun 27. J Exp Bot. 2007. PMID: 16804056 Review.
Cited by
-
A hydrophobic proline-rich motif is involved in the intracellular targeting of temperature-induced lipocalin.Plant Mol Biol. 2015 Jun;88(3):301-11. doi: 10.1007/s11103-015-0326-x. Epub 2015 May 10. Plant Mol Biol. 2015. PMID: 25957952 Free PMC article.
-
Fluorescent protein tagging as a tool to define the subcellular distribution of proteins in plants.Front Plant Sci. 2013 Jun 24;4:214. doi: 10.3389/fpls.2013.00214. eCollection 2013. Front Plant Sci. 2013. PMID: 23805149 Free PMC article.
-
Purification and functional characterization of protoplasts and intact vacuoles from grape cells.BMC Res Notes. 2010 Jan 22;3:19. doi: 10.1186/1756-0500-3-19. BMC Res Notes. 2010. PMID: 20181000 Free PMC article.
-
Microautophagy Mediates Vacuolar Delivery of Storage Proteins in Maize Aleurone Cells.Front Plant Sci. 2022 Feb 18;13:833612. doi: 10.3389/fpls.2022.833612. eCollection 2022. Front Plant Sci. 2022. PMID: 35251104 Free PMC article.
-
Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters.Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21187-92. doi: 10.1073/pnas.1013964107. Epub 2010 Nov 15. Proc Natl Acad Sci U S A. 2010. PMID: 21078981 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

