Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought
- PMID: 17151096
- PMCID: PMC1797597
- DOI: 10.1128/JVI.01459-06
Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought
Abstract
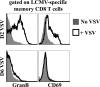
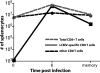
Measuring the magnitudes and specificities of antiviral CD8 T-cell responses is critical for understanding the dynamics and regulation of adaptive immunity. Despite many excellent studies, the accurate measurement of the total CD8 T-cell response directed against a particular infection has been hampered by an incomplete knowledge of all CD8 T-cell epitopes and also by potential contributions of bystander expansion among CD8 T cells of irrelevant specificities. Here, we use several techniques to provide a more complete accounting of the CD8 T-cell response generated upon infection of C57BL/6 mice with lymphocytic choriomeningitis virus (LCMV). Eight days following infection, we found that 85 to 95% of CD8 T cells exhibit an effector phenotype as indicated by granzyme B, 1B11, CD62L, CD11a, and CD127 expression. We demonstrate that CD8 T-cell expansion is due to cells that divide >7 times, whereas heterologous viral infections only elicited <3 divisions among bystander memory CD8 T cells. Furthermore, we found that approximately 80% of CD8 T cells in spleen were specific for ten different LCMV-derived epitopes at the peak of primary infection. These data suggest that following a single LCMV infection, effector CD8 T cells divide > or =15 times and account for at least 80%, and possibly as much as 95%, of the CD8 T-cell pool. Moreover, the response targeted a very broad array of peptide major histocompatibility complexes (MHCs), even though we examined epitopes derived from only two of the four proteins encoded by the LCMV genome and C57BL/6 mice only have two MHC class I alleles. These data illustrate the potential enormity, specificity, and breadth of CD8 T-cell responses to viral infection and demonstrate that bystander activation does not contribute to CD8 T-cell expansion.
Figures


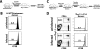

References
-
- Adams, A. B., M. A. Williams, T. R. Jones, N. Shirasugi, M. M. Durham, S. M. Kaech, E. J. Wherry, T. Onami, J. G. Lanier, K. E. Kokko, T. C. Pearson, R. Ahmed, and C. P. Larsen. 2003. Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Investig. 111:1887-1895. - PMC - PubMed
-
- Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. - PMC - PubMed
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Andersson, E. C., J. P. Christensen, A. Scheynius, O. Marker, and A. R. Thomsen. 1995. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand. J. Immunol. 42:110-118. - PubMed
-
- Andreasen, S. O., J. P. Christensen, O. Marker, and A. R. Thomsen. 1999. Virus-induced non-specific signals cause cell cycle progression of primed CD8(+) T cells but do not induce cell differentiation. Int. Immunol. 11:1463-1473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

