Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth
- PMID: 17151097
- PMCID: PMC1797585
- DOI: 10.1128/JVI.01420-06
Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth
Abstract
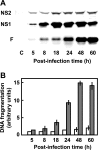
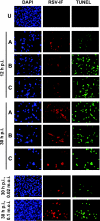
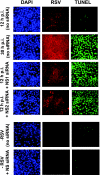
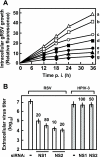
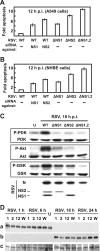
The two nonstructural (NS) proteins NS1 and NS2 of respiratory syncytial virus (RSV) are abundantly expressed in the infected cell but are not packaged in mature progeny virions. We found that both proteins were expressed early in infection, whereas the infected cells underwent apoptosis much later. Coincident with NS protein expression, a number of cellular antiapoptotic factors were expressed or activated at early stages, which included NF-kappaB and phosphorylated forms of protein kinases AKT, phosphoinositide-dependent protein kinase, and glycogen synthase kinase. Using specific short interfering RNAs (siRNAs), we achieved significant knockdown of one or both NS proteins in the infected cell, which resulted in abrogation of the antiapoptotic functions and led to early apoptosis. NS-dependent suppression of apoptosis was observed in Vero cells that are naturally devoid of type I interferons (IFN). The siRNA-based results were confirmed by the use of NS-deleted RSV mutants. Early activation of epidermal growth factor receptor (EGFR) in the RSV-infected cell did not require NS proteins. Premature apoptosis triggered by the loss of NS or by apoptosis-promoting drugs caused a severe reduction of RSV growth. Finally, recombinantly expressed NS1 and NS2, individually and together, reduced apoptosis by tumor necrosis factor alpha, suggesting an intrinsic antiapoptotic property of both. We conclude that the early-expressed nonstructural proteins of RSV boost viral replication by delaying the apoptosis of the infected cell via a novel IFN- and EGFR-independent pathway.
Figures




References
-
- Barik, S. 2004. Control of nonsegmented negative-strand RNA virus replication by siRNA. Virus Res. 102:27-35. - PubMed
-
- Barik, S. 2004. Development of gene-specific double-stranded RNA drugs. Ann. Med. 36:540-551. - PubMed
-
- Bitko, V., and S. Barik. 2001. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 80:441-454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

