CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1
- PMID: 17151103
- PMCID: PMC1865928
- DOI: 10.1128/JVI.01970-06
CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1
Abstract
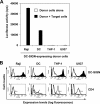
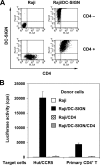
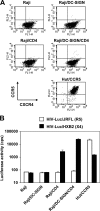
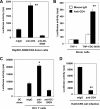
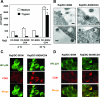
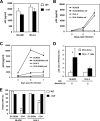
Dendritic cells (DCs) potently stimulate the cell-cell transmission of human immunodeficiency virus type 1 (HIV-1). However, the mechanisms that underlie DC transmission of HIV-1 to CD4(+) T cells are not fully understood. DC-SIGN, a C-type lectin, efficiently promotes HIV-1 trans infection. DC-SIGN is expressed in monocyte-derived DCs (MDDCs), macrophage subsets, activated B lymphocytes, and various mucosal tissues. MDDC-mediated HIV-1 transmission to CD4(+) T cells involves DC-SIGN-dependent and -independent mechanisms. DC-SIGN transmission of HIV-1 depends on the donor cell type. HIV-1 Nef can upregulate DC-SIGN expression and promote DC-T-cell clustering and HIV-1 spread. Nef also downregulates CD4 expression; however, the effect of the CD4 downmodulation on DC-mediated HIV-1 transmission has not been examined. Here, we report that CD4 expression levels correlate with inefficient HIV-1 transmission by monocytic cells expressing DC-SIGN. Expression of CD4 on Raji B cells strongly impaired DC-SIGN-mediated HIV-1 transmission to T cells. By contrast, enhanced HIV-1 transmission was observed when CD4 molecules on MDDCs and DC-SIGN-CD4-expressing cell lines were blocked with specific antibodies. Coexpression of CD4 and DC-SIGN in Raji cells promoted the internalization and intracellular retention of HIV-1. Interestingly, internalized HIV-1 particles were sorted and confined to late endosomal compartments that were positive for CD63 and CD81. Furthermore, in HIV-1-infected MDDCs, significant downregulation of CD4 by Nef expression correlated with enhanced viral transmission. These results suggest that CD4, which is present at various levels in DC-SIGN-positive primary cells, is a key regulator of HIV-1 transmission.
Figures

Similar articles
-
Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN.J Virol. 2007 Mar;81(5):2519-23. doi: 10.1128/JVI.01661-06. Epub 2006 Dec 20. J Virol. 2007. PMID: 17182696 Free PMC article.
-
Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells.J Virol. 2006 Mar;80(6):2949-57. doi: 10.1128/JVI.80.6.2949-2957.2006. J Virol. 2006. PMID: 16501104 Free PMC article.
-
Capture and transfer of simian immunodeficiency virus by macaque dendritic cells is enhanced by DC-SIGN.J Virol. 2002 Dec;76(23):11827-36. doi: 10.1128/jvi.76.23.11827-11836.2002. J Virol. 2002. PMID: 12414925 Free PMC article.
-
DC-SIGN points the way to a novel mechanism for HIV-1 transmission.MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review.
-
The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination?Expert Opin Ther Targets. 2002 Aug;6(4):423-31. doi: 10.1517/14728222.6.4.423. Expert Opin Ther Targets. 2002. PMID: 12223058 Review.
Cited by
-
HIV-1 increases TLR responses in human primary astrocytes.Sci Rep. 2015 Dec 16;5:17887. doi: 10.1038/srep17887. Sci Rep. 2015. PMID: 26671458 Free PMC article.
-
Macropinocytosis and cytoskeleton contribute to dendritic cell-mediated HIV-1 transmission to CD4+ T cells.Virology. 2008 Nov 10;381(1):143-54. doi: 10.1016/j.virol.2008.08.028. Epub 2008 Sep 18. Virology. 2008. PMID: 18804253 Free PMC article.
-
Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy.Nat Rev Microbiol. 2007 Aug;5(8):583-97. doi: 10.1038/nrmicro1707. Nat Rev Microbiol. 2007. PMID: 17632570 Free PMC article. Review.
-
N6-Methyladenosine-binding proteins suppress HIV-1 infectivity and viral production.J Biol Chem. 2018 Aug 24;293(34):12992-13005. doi: 10.1074/jbc.RA118.004215. Epub 2018 Jul 5. J Biol Chem. 2018. PMID: 29976753 Free PMC article.
-
Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry.Virology. 2008 Jun 5;375(2):442-51. doi: 10.1016/j.virol.2008.01.044. Epub 2008 Mar 10. Virology. 2008. PMID: 18329684 Free PMC article.
References
-
- Arrighi, J. F., M. Pion, M. Wiznerowicz, T. B. Geijtenbeek, E. Garcia, S. Abraham, F. Leuba, V. Dutoit, O. Ducrey-Rundquist, Y. van Kooyk, D. Trono, and V. Piguet. 2004. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 78:10848-10855. - PMC - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
-
- Bentham, M., S. Mazaleyrat, and M. Harris. 2003. The di-leucine motif in the cytoplasmic tail of CD4 is not required for binding to human immunodeficiency virus type 1 Nef, but is critical for CD4 down-modulation. J. Gen. Virol. 84:2705-2713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

