Murine MusD retrotransposon: structure and molecular evolution of an "intracellularized" retrovirus
- PMID: 17151128
- PMCID: PMC1797557
- DOI: 10.1128/JVI.02051-06
Murine MusD retrotransposon: structure and molecular evolution of an "intracellularized" retrovirus
Abstract
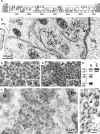
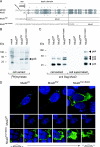
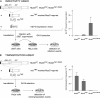
We had previously identified active autonomous copies of the MusD long terminal repeat-retrotransposon family, which have retained transpositional activity. These elements are closely related to betaretroviruses but lack an envelope (env) gene. Here we show that these elements encode strictly intracellular virus-like particles that can unambiguously be identified by electron microscopy. We demonstrate intracellular maturation of the particles, with a significant proportion of densely packed cores for wild-type MusD but not for a protease mutant. We show that the molecular origin of this unexpected intracellular localization is solely dependent on the N-terminal part of the Gag protein, which lacks a functional sequence for myristoylation and plasma membrane targeting: replacement of the N-terminal domain of the MusD matrix protein by that of its closest relative-the Mason-Pfizer monkey virus-led to targeting of the MusD Gag to the plasma membrane, with viral particles budding and being released into the cell supernatant. These particles can further be pseudotyped with a heterologous envelope protein and become infectious, thus "reconstituting" a functional retrovirus prone to proviral insertions. Consistent with its retroviral origin, a sequence with a constitutive transport element-like activity can further be identified at the MusD 3' untranslated region. A molecular scenario is proposed that accounts for the transition, during evolution, from an ancestral infectious betaretrovirus to the strictly intracellular MusD retrotransposon, involving not only the loss of the env gene but also an inability to escape the cell--via altered targeting of the Gag protein--resulting de facto in the generation of a very successful "intracellularized" insertional mutagen.
Figures



References
-
- Barbulescu, M., G. Turner, M. I. Seaman, A. S. Deinard, K. K. Kidd, and J. Lenz. 1999. Many human endogenous retrovirus K (HERV-K) proviruses are unique to humans. Curr. Biol. 9:861-868. - PubMed
-
- Baust, C., G. J. Baillie, and D. L. Mager. 2002. Insertional polymorphisms of ETn retrotransposons include a disruption of the wiz gene in C57BL/6 mice. Mamm. Genome 13:423-428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

