Homeologous recombination plays a major role in chromosome rearrangements that occur during meiosis of Brassica napus haploids
- PMID: 17151256
- PMCID: PMC1800630
- DOI: 10.1534/genetics.106.062968
Homeologous recombination plays a major role in chromosome rearrangements that occur during meiosis of Brassica napus haploids
Abstract
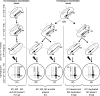
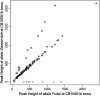
Chromosomal rearrangements can be triggered by recombination between distinct but related regions. Brassica napus (AACC; 2n = 38) is a recent allopolyploid species whose progenitor genomes are widely replicated. In this article, we analyze the extent to which chromosomal rearrangements originate from homeologous recombination during meiosis of haploid B. napus (n = 19) by genotyping progenies of haploid x euploid B. napus with molecular markers. Our study focuses on three pairs of homeologous regions selected for their differing levels of divergence (N1/N11, N3/N13, and N9/N18). We show that a high number of chromosomal rearrangements occur during meiosis of B. napus haploid and are transmitted by first division restitution (FDR)-like unreduced gametes to their progeny; half of the progeny of Darmor-bzh haploids display duplications and/or losses in the chromosomal regions being studied. We demonstrate that half of these rearrangements are due to recombination between regions of primary homeology, which represents a 10- to 100-fold increase compared to the frequency of homeologous recombination measured in euploid lines. Some of the other rearrangements certainly result from recombination between paralogous regions because we observed an average of one to two autosyndetic A-A and/or C-C bivalents at metaphase I of the B. napus haploid. These results are discussed in the context of genome evolution of B. napus.
Figures







Similar articles
-
Chromosome 'speed dating' during meiosis of polyploid Brassica hybrids and haploids.Cytogenet Genome Res. 2008;120(3-4):331-8. doi: 10.1159/000121082. Epub 2008 May 23. Cytogenet Genome Res. 2008. PMID: 18504362 Review.
-
The first meiosis of resynthesized Brassica napus, a genome blender.New Phytol. 2010 Apr;186(1):102-12. doi: 10.1111/j.1469-8137.2010.03182.x. Epub 2010 Feb 8. New Phytol. 2010. PMID: 20149113
-
Genetic regulation of meiotic cross-overs between related genomes in Brassica napus haploids and hybrids.Plant Cell. 2009 Feb;21(2):373-85. doi: 10.1105/tpc.108.062273. Epub 2009 Feb 3. Plant Cell. 2009. PMID: 19190241 Free PMC article.
-
Pairing and recombination at meiosis of Brassica rapa (AA) x Brassica napus (AACC) hybrids.Theor Appl Genet. 2006 Nov;113(8):1467-80. doi: 10.1007/s00122-006-0393-0. Epub 2006 Sep 16. Theor Appl Genet. 2006. PMID: 16983552
-
Homoeologous recombination in allopolyploids: the polyploid ratchet.New Phytol. 2010 Apr;186(1):18-28. doi: 10.1111/j.1469-8137.2009.03089.x. Epub 2009 Dec 1. New Phytol. 2010. PMID: 20002315 Review.
Cited by
-
Repeated polyploidy drove different levels of crossover suppression between homoeologous chromosomes in Brassica napus allohaploids.Plant Cell. 2010 Jul;22(7):2265-76. doi: 10.1105/tpc.109.072991. Epub 2010 Jul 16. Plant Cell. 2010. PMID: 20639447 Free PMC article.
-
Revisiting Meiosis in Sugarcane: Chromosomal Irregularities and the Prevalence of Bivalent Configurations.Front Genet. 2018 Jun 14;9:213. doi: 10.3389/fgene.2018.00213. eCollection 2018. Front Genet. 2018. PMID: 29963076 Free PMC article.
-
Cytogenetic evidence of mixed disomic and polysomic inheritance in an allotetraploid (AABB) Musa genotype.Ann Bot. 2012 Dec;110(8):1593-606. doi: 10.1093/aob/mcs220. Epub 2012 Oct 18. Ann Bot. 2012. PMID: 23087127 Free PMC article.
-
Long-read sequencing reveals widespread intragenic structural variants in a recent allopolyploid crop plant.Plant Biotechnol J. 2021 Feb;19(2):240-250. doi: 10.1111/pbi.13456. Epub 2020 Sep 6. Plant Biotechnol J. 2021. PMID: 32737959 Free PMC article.
-
Rapid Creation of Interspecific Hybrid Progeny to Broaden Genetic Distance through Double Haploid (DH) Inducer in Brassica napus.Plants (Basel). 2022 Mar 4;11(5):695. doi: 10.3390/plants11050695. Plants (Basel). 2022. PMID: 35270165 Free PMC article.
References
-
- Armstrong, K. C., and W. A. Keller, 1981. Chromosome pairing in haploids of Brassica campestris. Theor. Appl. Genet. 59: 49–52. - PubMed
-
- Armstrong, K. C., and W. A. Keller, 1982. Chromosome pairing in haploids of Brassica oleracea. Can. J. Genet. Cytol. 24: 735–739. - PubMed
-
- Attia, T., and G. Röbbelen, 1986. Meiotic pairing in haploids and amphihaploids of spontaneous versus synthetic origin in rape, Brassica napus L. Can. J. Genet. Cytol. 28: 330–334.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

