Plasticity compartments in basal dendrites of neocortical pyramidal neurons
- PMID: 17151275
- PMCID: PMC6674852
- DOI: 10.1523/JNEUROSCI.3502-06.2006
Plasticity compartments in basal dendrites of neocortical pyramidal neurons
Abstract
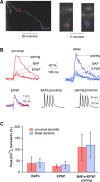
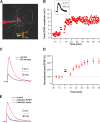
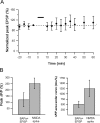
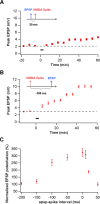
Synaptic plasticity rules widely determine how cortical networks develop and store information. Using confocal imaging and dual site focal synaptic stimulation, we show that basal dendrites, which receive the majority of synapses innervating neocortical pyramidal neurons, contain two compartments with respect to plasticity rules. Synapses innervating the proximal basal tree are easily modified when paired with the global activity of the neuron. In contrast, synapses innervating the distal basal tree fail to change in response to global suprathreshold activity or local dendritic spikes. These synapses can undergo long-term potentiation under unusual conditions when local NMDA spikes, which evoke large calcium transients, are paired with a "gating molecule," BDNF. Moreover, these synapses use a new temporal plasticity rule, which is an order of magnitude longer than spike timing dependent plasticity and prefers reversed presynaptic/postsynaptic activation order. The newly described plasticity compartmentalization of basal dendrites expands the networks plasticity rules and may support different learning and developmental functions.
Figures



Similar articles
-
Synaptic Plasticity Depends on the Fine-Scale Input Pattern in Thin Dendrites of CA1 Pyramidal Neurons.J Neurosci. 2020 Mar 25;40(13):2593-2605. doi: 10.1523/JNEUROSCI.2071-19.2020. Epub 2020 Feb 11. J Neurosci. 2020. PMID: 32047054 Free PMC article.
-
A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons.Neuron. 2006 Jul 20;51(2):227-38. doi: 10.1016/j.neuron.2006.06.017. Neuron. 2006. PMID: 16846857 Free PMC article.
-
Learning rules for spike timing-dependent plasticity depend on dendritic synapse location.J Neurosci. 2006 Oct 11;26(41):10420-9. doi: 10.1523/JNEUROSCI.2650-06.2006. J Neurosci. 2006. PMID: 17035526 Free PMC article.
-
A problem with Hebb and local spikes.Trends Neurosci. 2002 Sep;25(9):433-5. doi: 10.1016/s0166-2236(02)02200-2. Trends Neurosci. 2002. PMID: 12183194 Review.
-
Active properties of neocortical pyramidal neuron dendrites.Annu Rev Neurosci. 2013 Jul 8;36:1-24. doi: 10.1146/annurev-neuro-062111-150343. Annu Rev Neurosci. 2013. PMID: 23841837 Review.
Cited by
-
Unifying Long-Term Plasticity Rules for Excitatory Synapses by Modeling Dendrites of Cortical Pyramidal Neurons.Cell Rep. 2019 Dec 24;29(13):4295-4307.e6. doi: 10.1016/j.celrep.2019.11.068. Cell Rep. 2019. PMID: 31875541 Free PMC article.
-
Perceptron Learning and Classification in a Modeled Cortical Pyramidal Cell.Front Comput Neurosci. 2020 Apr 24;14:33. doi: 10.3389/fncom.2020.00033. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32390819 Free PMC article.
-
Bidirectional Hebbian Plasticity Induced by Low-Frequency Stimulation in Basal Dendrites of Rat Barrel Cortex Layer 5 Pyramidal Neurons.Front Cell Neurosci. 2017 Feb 1;11:8. doi: 10.3389/fncel.2017.00008. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28203145 Free PMC article.
-
Effect of the environment on the dendritic morphology of the rat auditory cortex.Synapse. 2010 Feb;64(2):97-110. doi: 10.1002/syn.20710. Synapse. 2010. PMID: 19771593 Free PMC article.
-
Embedded ensemble encoding hypothesis: The role of the "Prepared" cell.J Neurosci Res. 2018 Sep;96(9):1543-1559. doi: 10.1002/jnr.24240. Epub 2018 Apr 6. J Neurosci Res. 2018. PMID: 29633330 Free PMC article. Review.
References
-
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;10:433–437. - PubMed
-
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
-
- Bastian J, Chacron MJ, Maler L. Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron. 2004;41:767–779. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
