GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses
- PMID: 17151279
- PMCID: PMC2366897
- DOI: 10.1523/JNEUROSCI.4214-06.2006
GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses
Abstract
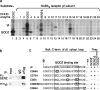
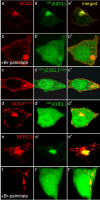
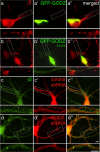
Golgi-specific DHHC (Asp-His-His-Cys) zinc finger protein (GODZ) is a DHHC family palmitoyl acyltransferase that is implicated in palmitoylation and regulated trafficking of diverse substrates that function either at inhibitory or excitatory synapses. Of particular interest is the gamma2 subunit of GABA(A) receptors, which is required for targeting these receptors to inhibitory synapses. Here, we report that GODZ and, to a lesser extent, its close paralog sertoli cell gene with a zinc finger domain-beta (SERZ-beta) are the main members of the DHHC family of enzymes that are able to palmitoylate the gamma2 subunit in heterologous cells. Yeast two-hybrid and colocalization assays in human embryonic kidney 293T (HEK293T) cells indicate that GODZ and SERZ-beta show indistinguishable palmitoylation-dependent interaction with the gamma2 subunit. After coexpression in HEK293T cells, they form homomultimers and heteromultimers, as shown by coimmunoprecipitation and in vivo cross-linking experiments. Analyses in neurons transfected with dominant-negative GODZ (GODZ(C157S)) or plasmid-based GODZ-specific RNAi indicate that GODZ is required for normal accumulation of GABA(A) receptors at synapses, for normal whole-cell and synaptic GABAergic inhibitory function and, indirectly, for GABAergic innervation. Unexpectedly, GODZ was found to be dispensable for normal postsynaptic AMPA receptor-mediated glutamatergic transmission. We conclude that GODZ-mediated palmitoylation of GABA(A) receptors and possibly other substrates contributes selectively to the formation and normal function of GABAergic inhibitory synapses.
Figures
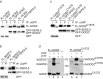


Similar articles
-
Dissociation of Golgi-associated DHHC-type Zinc Finger Protein (GODZ)- and Sertoli Cell Gene with a Zinc Finger Domain-β (SERZ-β)-mediated Palmitoylation by Loss of Function Analyses in Knock-out Mice.J Biol Chem. 2016 Dec 30;291(53):27371-27386. doi: 10.1074/jbc.M116.732768. Epub 2016 Nov 14. J Biol Chem. 2016. PMID: 27875292 Free PMC article.
-
The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ.J Neurosci. 2004 Jun 30;24(26):5881-91. doi: 10.1523/JNEUROSCI.1037-04.2004. J Neurosci. 2004. PMID: 15229235 Free PMC article.
-
Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin.J Neurosci. 2005 Jan 19;25(3):594-603. doi: 10.1523/JNEUROSCI.4011-04.2005. J Neurosci. 2005. PMID: 15659595 Free PMC article.
-
GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development.Curr Opin Neurobiol. 2008 Feb;18(1):77-83. doi: 10.1016/j.conb.2008.05.008. Epub 2008 May 29. Curr Opin Neurobiol. 2008. PMID: 18513949 Free PMC article. Review.
-
GABAA receptor trafficking-mediated plasticity of inhibitory synapses.Neuron. 2011 May 12;70(3):385-409. doi: 10.1016/j.neuron.2011.03.024. Neuron. 2011. PMID: 21555068 Free PMC article. Review.
Cited by
-
Homeostatic competition between phasic and tonic inhibition.J Biol Chem. 2013 Aug 30;288(35):25053-25065. doi: 10.1074/jbc.M113.491464. Epub 2013 Jul 9. J Biol Chem. 2013. PMID: 23839941 Free PMC article.
-
γ2 GABAAR Trafficking and the Consequences of Human Genetic Variation.Front Cell Neurosci. 2018 Aug 23;12:265. doi: 10.3389/fncel.2018.00265. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30190672 Free PMC article. Review.
-
Acylation - A New Means to Control Traffic Through the Golgi.Front Cell Dev Biol. 2019 Jun 12;7:109. doi: 10.3389/fcell.2019.00109. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31245373 Free PMC article. Review.
-
ZDHHC11 modulates innate immune response to DNA virus by mediating MITA-IRF3 association.Cell Mol Immunol. 2018 Oct;15(10):907-916. doi: 10.1038/cmi.2017.146. Epub 2018 Feb 12. Cell Mol Immunol. 2018. PMID: 29429998 Free PMC article.
-
Identification of G protein alpha subunit-palmitoylating enzyme.Mol Cell Biol. 2009 Jan;29(2):435-47. doi: 10.1128/MCB.01144-08. Epub 2008 Nov 10. Mol Cell Biol. 2009. PMID: 19001095 Free PMC article.
References
-
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. - PubMed
-
- Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
