Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation
- PMID: 17151286
- PMCID: PMC6674840
- DOI: 10.1523/JNEUROSCI.4937-05.2006
Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation
Abstract
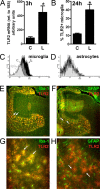
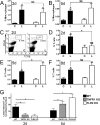
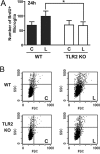
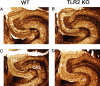
Reactive gliosis is a prominent feature of neurodegenerative and neuroinflammatory disease in the CNS, yet the stimuli that drive this response are not known. There is growing appreciation that signaling through Toll-like receptors (TLRs), which is key to generating innate responses to infection, may have pathogen-independent roles. We show that TLR2 was selectively upregulated by microglia in the denervated zones of the hippocampus in response to stereotactic transection of axons in the entorhinal cortex. In mice lacking TLR2, there were transient, selective reductions in lesion-induced expression of cytokines and chemokines. Recruitment of T cells, but not macrophages, was delayed in TLR2-deficient mice, as well as in mice lacking TNFR1 (tumor necrosis factor receptor 1). TLR2 deficiency also affected microglial proliferative expansion, whereas all of these events were unaffected in TLR4-mutant mice. Consistent with the fact that responses in knock-out mice had all returned to wild-type levels by 8 d, there was no evidence for effects on neuronal plasticity at 20 d. These results identify a role for TLR2 signaling in the early glial response to brain injury, acting as an innate bridge to neuroinflammation.
Figures



References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. - PubMed
-
- Babcock A, Owens T. Chemokines in experimental autoimmune encephalomyelitis and multiple sclerosis. Adv Exp Med Biol. 2003;520:120–132. - PubMed
-
- Bechmann I, Nitsch R. Involvement of non-neuronal cells in entorhinal-hippocampal reorganization following lesions. Ann NY Acad Sci. 2000;911:192–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
