Novel aspects of prions, their receptor molecules, and innovative approaches for TSE therapy
- PMID: 17151946
- PMCID: PMC11517296
- DOI: 10.1007/s10571-006-9121-1
Novel aspects of prions, their receptor molecules, and innovative approaches for TSE therapy
Abstract
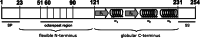
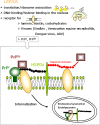
1. Prion diseases are a group of rare, fatal neurodegenerative diseases, also known as transmissible spongiform encephalopathies (TSEs), that affect both animals and humans and include bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep, chronic wasting disease (CWD) in deer and elk, and Creutzfeldt-Jakob disease (CJD) in humans. TSEs are usually rapidly progressive and clinical symptoms comprise dementia and loss of movement coordination due to the accumulation of an abnormal isoform (PrP(Sc)) of the host-encoded prion protein (PrP(c)). 2. This article reviews the current knowledge on PrP(c) and PrP(Sc), prion replication mechanisms, interaction partners of prions, and their cell surface receptors. Several strategies, summarized in this article, have been investigated for an effective antiprion treatment including development of a vaccination therapy and screening for potent chemical compounds. Currently, no effective treatment for prion diseases is available. 3. The identification of the 37 kDa/67 kDa laminin receptor (LRP/LR) and heparan sulfate as cell surface receptors for prions, however, opens new avenues for the development of alternative TSE therapies.
Figures
Similar articles
-
Therapeutic approaches targeting the prion receptor LRP/LR.Vet Microbiol. 2007 Aug 31;123(4):387-93. doi: 10.1016/j.vetmic.2007.04.005. Epub 2007 Apr 8. Vet Microbiol. 2007. PMID: 17498894 Review.
-
Prion interaction with the 37-kDa/67-kDa laminin receptor on enterocytes as a cellular model for intestinal uptake of prions.J Mol Biol. 2010 Sep 17;402(2):293-300. doi: 10.1016/j.jmb.2010.06.055. Epub 2010 Jul 13. J Mol Biol. 2010. PMID: 20603132
-
Role of the 37 kDa laminin receptor precursor in the life cycle of prions.Transfus Clin Biol. 1999 Feb;6(1):7-16. doi: 10.1016/S1246-7820(99)80006-8. Transfus Clin Biol. 1999. PMID: 10188208 Review.
-
The transmissible spongiform encephalopathies of livestock.ILAR J. 2015;56(1):7-25. doi: 10.1093/ilar/ilv008. ILAR J. 2015. PMID: 25991695 Review.
-
Prion protein self-interactions: a gateway to novel therapeutic strategies?Vaccine. 2010 Nov 16;28(49):7810-23. doi: 10.1016/j.vaccine.2010.09.012. Epub 2010 Oct 20. Vaccine. 2010. PMID: 20932496 Review.
Cited by
-
Prion propagation and toxicity occur in vitro with two-phase kinetics specific to strain and neuronal type.J Virol. 2013 Mar;87(5):2535-48. doi: 10.1128/JVI.03082-12. Epub 2012 Dec 19. J Virol. 2013. PMID: 23255799 Free PMC article.
-
The 37/67 kDa laminin receptor (LR) inhibitor, NSC47924, affects 37/67 kDa LR cell surface localization and interaction with the cellular prion protein.Sci Rep. 2016 Apr 13;6:24457. doi: 10.1038/srep24457. Sci Rep. 2016. PMID: 27071549 Free PMC article.
-
siRNA-mediated silencing of the 37/67-kDa high affinity laminin receptor in Hep3B cells induces apoptosis.Cell Mol Biol Lett. 2008;13(3):452-64. doi: 10.2478/s11658-008-0017-6. Epub 2008 Apr 18. Cell Mol Biol Lett. 2008. PMID: 18425431 Free PMC article.
-
Orally administered prion protein is incorporated by m cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice.Am J Pathol. 2011 Sep;179(3):1301-9. doi: 10.1016/j.ajpath.2011.05.058. Epub 2011 Jul 18. Am J Pathol. 2011. PMID: 21763679 Free PMC article.
-
Region-specific protein misfolding cyclic amplification reproduces brain tropism of prion strains.J Biol Chem. 2017 Oct 6;292(40):16688-16696. doi: 10.1074/jbc.M117.793646. Epub 2017 Aug 15. J Biol Chem. 2017. PMID: 28821618 Free PMC article.
References
-
- Achour, A. (2002). Phenothiazines and prion diseases: A potential mechanism of action towards oxidative stress. Int. J. Antimicrob. Agents20:305–306. - PubMed
-
- Adjou, K. T., Simoneau, S., Sales, N., Lamoury, F., Dormont, D., Papy-Garcia, D., Barritault, D., Deslys, J. P., and Lasmezas, C. I. (2003). A novel generation of heparan sulfate mimetics for the treatment of prion diseases. J. Gen. Virol.84(Pt. 9):2595–2603. - PubMed
-
- Alper, T., Cramp, W. A., Haig, D. A., and Clarke, M. C. (1967). Does the agent of scrapie replicate without nucleic acid? Nature214(90):764–766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

