Importance of force linkage in mechanochemistry of adhesion receptors
- PMID: 17154539
- PMCID: PMC1766327
- DOI: 10.1021/bi061566o
Importance of force linkage in mechanochemistry of adhesion receptors
Abstract
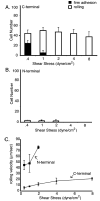
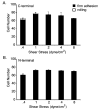
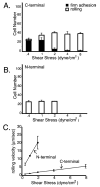
The alpha subunit-inserted (I) domain of integrin alphaLbeta2 [lymphocyte function-associated antigen-1 (LFA-1)] binds to intercellular adhesion molecule-1 (ICAM-1). The C- and N-termini of the alpha I domain are near one another on the "lower" face, opposite the metal ion-dependent adhesion site (MIDAS) on the "upper face". In conversion to the open alpha I domain conformation, a 7 A downward, axial displacement of C-terminal helix alpha7 is allosterically linked to rearrangement of the MIDAS into its high-affinity conformation. Here, we test the hypothesis that when an applied force is appropriately linked to conformational change, the conformational change can stabilize adhesive interactions that resist the applied force. Integrin alpha I domains were anchored to the cell surface through their C- or N-termini using type I or II transmembrane domains, respectively. C-terminal but not N-terminal anchorage robustly supported cell rolling on ICAM-1 substrates in shear flow. In contrast, when the alphaL I domain was mutationally stabilized in the open conformation with a disulfide bond, it mediated comparable levels of firm adhesion with type I and type II membrane anchors. To exclude other effects as the source of differential adhesion, these results were replicated using alpha I domains conjugated through the N- or C-terminus to polystyrene microspheres. Our results demonstrate a mechanical feedback system for regulating the strength of an adhesive bond. A review of crystal structures of integrin alpha and beta subunit I domains and selectins in high- and low-affinity conformations demonstrates a common mechanochemical design in which biologically applied tensile force stabilizes the more extended, high-affinity conformation.
Figures



References
-
- Zhu C, Long M, Chesla SE, Bongrand P. Measuring receptor/ligand interaction at the single-bond level: Experimental and interpretative issues. Ann Biomed Eng. 2002;30:305–314. - PubMed
-
- Konstantopoulos K, Hanley WD, Wirtz D. Receptor-ligand binding: ‘Catch’ bonds finally caught. Curr Biol. 2003;13:R611–R613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

