A comparison of the binding of secretory component to immunoglobulin A (IgA) in human colostral S-IgA1 and S-IgA2
- PMID: 17156102
- PMCID: PMC2265853
- DOI: 10.1111/j.1365-2567.2006.02498.x
A comparison of the binding of secretory component to immunoglobulin A (IgA) in human colostral S-IgA1 and S-IgA2
Abstract
A detailed investigation of the binding of secretory component to immunoglobulin A (IgA) in human secretory IgA2 (S-IgA2) was made possible by the development of a new method of purifying S-IgA1, S-IgA2 and free secretory component from human colostrum using thiophilic gel chromatography and chromatography on Jacalin-agarose. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of unreduced pure S-IgA2 revealed that, unlike in S-IgA1, a significant proportion of the secretory component was bound non-covalently in S-IgA2. When S-IgA1 was incubated with a protease purified from Proteus mirabilis the secretory component, but not the alpha-chain, was cleaved. This is in contrast to serum IgA1, in which the alpha-chain was cleaved under the same conditions - direct evidence that secretory component does protect the alpha-chain from proteolytic cleavage in S-IgA. Comparisons between the products of cleavage with P. mirabilis protease of free secretory component and bound secretory component in S-IgA1 and S-IgA2 also indicated that, contrary to the general assumption, the binding of secretory component to IgA is different in S-IgA2 from that in S-IgA1.
Figures
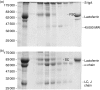



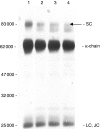

References
-
- Woof JM, Kerr MA, Moro I, Mestecky JR. Mucosal immunoglobulins. In: Mestecky JR, Bienenstock J, Lamm ME, Mayer L, Mcghee JR, Strober W, editors. Mucosal Immunology. 3. San Diego: Academic Press Inc.; 2005. pp. 153–81.
-
- Woof JM, Kerr MA. Function of immunoglobulin A in immunity. J Pathol. 2006;208:270–82. - PubMed
-
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. - PubMed
-
- Fallgreen-Gebauer E, Gebauer W, Bastian A, Kratzin HD, Eiffert H, Zimmermann B, Karas M, Hilschmann N. The covalent linkage of secretory component to IgA. Structure of sIgA. Biol Chem Hoppe Seyler. 1993;374:1023–8. - PubMed
-
- Chintalacharuvu KR, Tavill AS, Louis LN, Vaerman JP, Lamm ME, Kaetzel CS. Disulfide bond formation between dimeric immunoglobulin A and the polymeric immunoglobulin receptor during hepatic transcytosis. Hepatology. 1994;19:162–73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

