Effectiveness of smoking cessation therapies: a systematic review and meta-analysis
- PMID: 17156479
- PMCID: PMC1764891
- DOI: 10.1186/1471-2458-6-300
Effectiveness of smoking cessation therapies: a systematic review and meta-analysis
Abstract
Background: Smoking remains the leading preventable cause of premature deaths. Several pharmacological interventions now exist to aid smokers in cessation. These include Nicotine Replacement Therapy [NRT], bupropion, and varenicline. We aimed to assess their relative efficacy in smoking cessation by conducting a systematic review and meta-analysis.
Methods: We searched 10 electronic medical databases (inception to Sept. 2006) and bibliographies of published reviews. We selected randomized controlled trials [RCTs] evaluating interventions for smoking cessation at 1 year, through chemical confirmation. Our primary endpoint was smoking cessation at 1 year. Secondary endpoints included short-term smoking cessation (approximately 3 months) and adverse events. We conducted random-effects meta-analysis and meta-regression. We compared treatment effects across interventions using head-to-head trials and when these did not exist, we calculated indirect comparisons.
Results: We identified 70 trials of NRT versus control at 1 year, Odds Ratio [OR] 1.71, 95% Confidence Interval [CI], 1.55-1.88, P =< 0.0001). This was consistent when examining all placebo-controlled trials (49 RCTs, OR 1.78, 95% CI, 1.60-1.99), NRT gum (OR 1.60, 95% CI, 1.37-1.86) or patch (OR 1.63, 95% CI, 1.41-1.89). NRT also reduced smoking at 3 months (OR 1.98, 95% CI, 1.77-2.21). Bupropion trials were superior to controls at 1 year (12 RCTs, OR1.56, 95% CI, 1.10-2.21, P = 0.01) and at 3 months (OR 2.13, 95% CI, 1.72-2.64). Two RCTs evaluated the superiority of bupropion versus NRT at 1 year (OR 1.14, 95% CI, 0.20-6.42). Varenicline was superior to placebo at 1 year (4 RCTs, OR 2.96, 95% CI, 2.12-4.12, P =< 0.0001) and also at approximately 3 months (OR 3.75, 95% CI, 2.65-5.30). Three RCTs evaluated the effectiveness of varenicline versus bupropion at 1 year (OR 1.58, 95% CI, 1.22-2.05) and at approximately 3 months (OR 1.61, 95% CI, 1.16-2.21). Using indirect comparisons, varenicline was superior to NRT when compared to placebo controls (OR 1.66, 95% CI 1.17-2.36, P = 0.004) or to all controls at 1 year (OR 1.73, 95% CI 1.22-2.45, P = 0.001). This was also the case for 3-month data. Adverse events were not systematically different across studies.
Conclusion: NRT, bupropion and varenicline all provide therapeutic effects in assisting with smoking cessation. Direct and indirect comparisons identify a hierarchy of effectiveness.
Figures



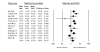


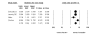
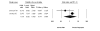
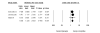
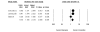
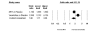
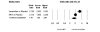
References
-
- Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. - PubMed
-
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004:CD000146. - PubMed
-
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2004:CD000031. - PubMed
-
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

