Murine coronavirus-induced oligodendrocyte apoptosis is mediated through the activation of the Fas signaling pathway
- PMID: 17156812
- PMCID: PMC1851929
- DOI: 10.1016/j.virol.2006.10.044
Murine coronavirus-induced oligodendrocyte apoptosis is mediated through the activation of the Fas signaling pathway
Abstract
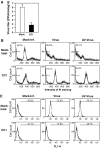
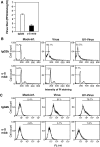
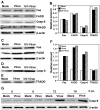
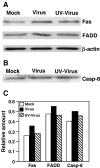
We previously showed that infection of rat oligodendrocytes by ultraviolet light-inactivated mouse hepatitis virus (MHV) resulted in apoptosis, suggesting that the apoptosis is triggered during cell entry. To further characterize the earliest apoptotic signaling events, here we treated cells with an antibody specific to the MHV receptor prior to and during virus infection or with an antibody specific to MHV spike protein following virus binding. Both treatments blocked virus infection and apoptosis, indicating that virus-receptor binding is necessary but not sufficient for the apoptosis induction. Furthermore, virus infection significantly increased the formation of the "death-receptor complexes" consisting of Fas, Fas-associated death domain and procaspase-8, but did not induce the complexes involving the tumor necrosis factor receptor and its associated death domain, demonstrating the specific activation of the Fas signaling pathway. Moreover, virus infection did not alter the abundance of the individual proteins of the complexes, suggesting that the activation of the Fas signaling pathway was at the post-translational level. Treatment with a Fas/Fc chimera, which blocks Fas-Fas ligand-mediated apoptosis, inhibited the formation of the complexes and blocked the activation of caspase-8 and apoptosis in MHV-infected cells. It also inhibited the release of cytochrome c from mitochondria and the activation of caspase-9. These results demonstrate that oligodendrocyte apoptosis is triggered by MHV infection during cell entry through the activation of the Fas signaling pathway.
Figures


Similar articles
-
Induction of caspase-dependent apoptosis in cultured rat oligodendrocytes by murine coronavirus is mediated during cell entry and does not require virus replication.J Virol. 2003 Nov;77(22):11952-63. doi: 10.1128/jvi.77.22.11952-11963.2003. J Virol. 2003. PMID: 14581532 Free PMC article.
-
Role of the mitochondrial signaling pathway in murine coronavirus-induced oligodendrocyte apoptosis.J Virol. 2006 Jan;80(1):395-403. doi: 10.1128/JVI.80.1.395-403.2006. J Virol. 2006. PMID: 16352564 Free PMC article.
-
Murine coronavirus-induced apoptosis in 17Cl-1 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and bid cleavage.Virology. 2002 Oct 25;302(2):321-32. doi: 10.1006/viro.2002.1626. Virology. 2002. PMID: 12441076 Free PMC article.
-
Regulation of CD95/Fas signaling at the DISC.Cell Death Differ. 2012 Jan;19(1):36-41. doi: 10.1038/cdd.2011.155. Epub 2011 Nov 11. Cell Death Differ. 2012. PMID: 22075988 Free PMC article. Review.
-
Live and let die: regulatory mechanisms in Fas-mediated apoptosis.Cell Signal. 2003 Nov;15(11):983-92. doi: 10.1016/s0898-6568(03)00093-7. Cell Signal. 2003. PMID: 14499341 Review.
Cited by
-
The chemokine receptor CXCR2 and coronavirus-induced neurologic disease.Virology. 2013 Jan 5;435(1):110-7. doi: 10.1016/j.virol.2012.08.049. Virology. 2013. PMID: 23217621 Free PMC article. Review.
-
In toxic demyelination oligodendroglial cell death occurs early and is FAS independent.Neurobiol Dis. 2010 Feb;37(2):362-9. doi: 10.1016/j.nbd.2009.10.016. Epub 2009 Oct 22. Neurobiol Dis. 2010. PMID: 19853662 Free PMC article.
-
Bid cleavage, cytochrome c release and caspase activation in canine coronavirus-induced apoptosis.Vet Microbiol. 2010 Feb 24;141(1-2):36-45. doi: 10.1016/j.vetmic.2009.09.001. Epub 2009 Sep 11. Vet Microbiol. 2010. PMID: 19781871 Free PMC article.
-
Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity.J Virol. 2009 Mar;83(5):2298-309. doi: 10.1128/JVI.01245-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109397 Free PMC article.
-
Animal Coronaviruses Induced Apoptosis.Life (Basel). 2021 Feb 26;11(3):185. doi: 10.3390/life11030185. Life (Basel). 2021. PMID: 33652685 Free PMC article. Review.
References
-
- Barac-Latas V., Suchanek G., Breitschopf H., Stuehler A., Wege H., Lassmann H. Patterns of oligodendrocyte pathology in coronavirus-induced subacute demyelinating encephalomyelitis in the Lewis rat. Glia. 1997;19:1–12. - PubMed
-
- Cheng J., Zhou T., Liu C., Shapiro J.P., Brauer M.J., Kiefer M.C., Barr P.J., Mountz J.D. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

