Nerve injury-induced tactile allodynia is present in the absence of FOS labeling in retrogradely labeled post-synaptic dorsal column neurons
- PMID: 17156921
- PMCID: PMC4028680
- DOI: 10.1016/j.pain.2006.10.009
Nerve injury-induced tactile allodynia is present in the absence of FOS labeling in retrogradely labeled post-synaptic dorsal column neurons
Abstract
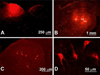
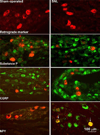
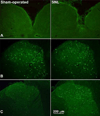
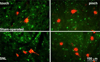
The dorsal column pathway consists of direct projections from primary afferents and of ascending fibers of the post-synaptic dorsal column (PSDC) cells. This pathway mediates touch but may also mediate allodynia after nerve injury. The role of PSDC neurons in nerve injury-induced mechanical allodynia is unknown. Repetitive gentle, tactile stimulus or noxious pinch was applied to the ipsilateral hindpaw of rats with spinal nerve ligation (SNL) or sham surgery that had previously received tetramethylrhodamine dextran in the ipsilateral n. gracilis. Both touch and noxious stimuli produced marked increases in FOS expression in other cells throughout all laminae of the ipsilateral dorsal horn after nerve injury. However, virtually none of the identified PSDC cells expressed FOS immunofluorescence in response to repetitive touch or pinch in either the nerve-injured or sham groups. In contrast, labeled PSDC cells expressed FOS in response to ureter ligation and labeled spinothalamic tract (STT) cells expressed FOS in response to noxious pinch. Identified PSDC neurons from either sham-operated or SNL rats did not express immunoreactivity to substance P, CGRP, NPY, PKCY, MOR, the NK1 and the NPY-Y1 receptor. Retrogradely labeled DRG cells of nerve injured rats were large diameter neurons, which expressed NPY, but no detectable CGRP or substance P. Spinal nerve injury sensitizes neurons in the spinal dorsal horn to repetitive light touch but PSDC neurons apparently do not participate in touch-evoked allodynia. Sensitization of these non-PSDC neurons may result in activation of projections integral to the spinal/supraspinal processing of enhanced pain states and of descending facilitation, thus priming the central nervous system to interpret tactile stimuli as being aversive.
Figures
Similar articles
-
Fos expression in spinothalamic and postsynaptic dorsal column neurons following noxious visceral and cutaneous stimuli.Pain. 2003 Jul;104(1-2):249-57. doi: 10.1016/s0304-3959(03)00013-7. Pain. 2003. PMID: 12855335
-
Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections.Pain. 2001 Feb 1;90(1-2):105-11. doi: 10.1016/s0304-3959(00)00392-4. Pain. 2001. PMID: 11166976
-
Somatic noxious mechanical stimulation induces Fos expression in the postsynaptic dorsal column neurons in laminae III and IV of the rat spinal dorsal horn.Neurosci Res. 2001 Aug;40(4):343-50. doi: 10.1016/s0168-0102(01)00245-0. Neurosci Res. 2001. PMID: 11463480
-
Fos, nociception and the dorsal horn.Prog Neurobiol. 2005 Dec;77(5):299-352. doi: 10.1016/j.pneurobio.2005.11.002. Epub 2005 Dec 13. Prog Neurobiol. 2005. PMID: 16356622 Review.
-
Effects of general anesthetics on visceral pain transmission in the spinal cord.Mol Pain. 2008 Oct 30;4:50. doi: 10.1186/1744-8069-4-50. Mol Pain. 2008. PMID: 18973669 Free PMC article. Review.
Cited by
-
Descending facilitation maintains long-term spontaneous neuropathic pain.J Pain. 2013 Aug;14(8):845-53. doi: 10.1016/j.jpain.2013.02.011. Epub 2013 Apr 19. J Pain. 2013. PMID: 23602267 Free PMC article.
-
Effects of general anesthetics on substance P release and c-Fos expression in the spinal dorsal horn.Anesthesiology. 2013 Aug;119(2):433-42. doi: 10.1097/ALN.0b013e31829996b6. Anesthesiology. 2013. PMID: 23708866 Free PMC article.
-
Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain.Neuroscience. 2009 Apr 21;160(1):174-85. doi: 10.1016/j.neuroscience.2009.02.023. Epub 2009 Feb 14. Neuroscience. 2009. PMID: 19223010 Free PMC article.
-
Pregnancy suppresses neuropathic pain induced by chronic constriction injury in rats through the inhibition of TNF-α.J Pain Res. 2017 Mar 8;10:567-574. doi: 10.2147/JPR.S121810. eCollection 2017. J Pain Res. 2017. PMID: 28331359 Free PMC article.
-
Spatiotemporal changes in NSF expression of DRG neurons in a rat model of spinal nerve ligation.J Mol Neurosci. 2014 Aug;53(4):645-53. doi: 10.1007/s12031-014-0231-9. Epub 2014 Jan 19. J Mol Neurosci. 2014. PMID: 24443234
References
-
- Al-Chaer ED, Feng Y, Willis WD. Comparative study of viscerosomatic input onto postsynaptic dorsal column and spinothalamic tract neurons in the primate. J Neurophysiol. 1999;82:1876–1882. - PubMed
-
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996a;76:2675–2690. - PubMed
-
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol. 1996b;76:2661–2674. - PubMed
-
- Avelino A, Cruz F, Coimbra A. Sites of renal pain processing in the rat spinal cord. A c-fos study using a percutaneous method to perform ureteral obstruction. J Auton Nerv Syst. 1997;67:60–66. - PubMed
-
- Bennett GJ, Nishikawa N, Lu GW, Hoffert MJ, Dubner R. The morphology of dorsal column postsynaptic spinomedullary neurons in the cat. J Comp Neurol. 1984;224:568–578. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

