Calcium permeable AMPA receptors and autoreceptors in external tufted cells of rat olfactory bulb
- PMID: 17156930
- PMCID: PMC2094052
- DOI: 10.1016/j.neuroscience.2006.10.041
Calcium permeable AMPA receptors and autoreceptors in external tufted cells of rat olfactory bulb
Abstract
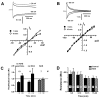
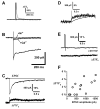
Glomeruli are functional units of the olfactory bulb responsible for early processing of odor information encoded by single olfactory receptor genes. Glomerular neural circuitry includes numerous external tufted (ET) cells whose rhythmic burst firing may mediate synchronization of bulbar activity with the inhalation cycle. Bursting is entrained by glutamatergic input from olfactory nerve terminals, so specific properties of ionotropic glutamate receptors on ET cells are likely to be important determinants of olfactory processing. Particularly intriguing is recent evidence that AMPA receptors of juxta-glomerular neurons may permeate calcium. This could provide a novel pathway for regulating ET cell signaling. We tested the hypothesis that ET cells express functional calcium-permeable AMPA receptors. In rat olfactory bulb slices, excitatory postsynaptic currents (EPSCs) in ET cells were evoked by olfactory nerve shock, and by uncaging glutamate. We found attenuation of AMPA/kainate EPSCs by 1-naphthyl acetyl-spermine (NAS), an open-channel blocker specific for calcium permeable AMPA receptors. Cyclothiazide strongly potentiated EPSCs, indicating a major contribution from AMPA receptors. The current-voltage (I-V) relation of uncaging EPSCs showed weak inward rectification which was lost after > approximately 10 min of whole-cell dialysis, and was absent in NAS. In kainate-stimulated slices, Co(2+) ions permeated cells of the glomerular layer. Large AMPA EPSCs were accompanied by fluorescence signals in fluo-4 loaded cells, suggesting calcium permeation. Depolarizing pulses evoked slow tail currents with pharmacology consistent with involvement of calcium permeable AMPA autoreceptors. Tail currents were abolished by Cd(2+) and (+/-)-4-(4-aminophenyl)-2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), and were sensitive to NAS block. Glutamate autoreceptors were confirmed by uncaging intracellular calcium to evoke a large inward current. Our results provide evidence that calcium permeable AMPA receptors reside on ET cells, and are divided into at least two functionally distinct pools: postsynaptic receptors at olfactory nerve synaptic terminals, and autoreceptors sensitive to glutamate released from dendrodendritic synapses.
Figures

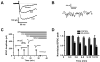


Similar articles
-
Evidence for low GluR2 AMPA receptor subunit expression at synapses in the rat basolateral amygdala.J Neurochem. 2005 Sep;94(6):1728-38. doi: 10.1111/j.1471-4159.2005.03334.x. Epub 2005 Jul 25. J Neurochem. 2005. PMID: 16045445 Free PMC article.
-
Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors.J Neurophysiol. 2000 Sep;84(3):1194-203. doi: 10.1152/jn.2000.84.3.1194. J Neurophysiol. 2000. PMID: 10979995
-
Activation of postsynaptic GABAB receptors modulates the bursting pattern and synaptic activity of olfactory bulb juxtaglomerular neurons.J Neurophysiol. 2008 Jan;99(1):308-19. doi: 10.1152/jn.01086.2007. Epub 2007 Nov 21. J Neurophysiol. 2008. PMID: 18032562 Free PMC article.
-
AMPA-type glutamate receptors--nonselective cation channels mediating fast excitatory transmission in the CNS.EXS. 1993;66:61-76. doi: 10.1007/978-3-0348-7327-7_4. EXS. 1993. PMID: 7505664 Review.
-
Helping thy neighbors: spillover at the mossy fiber glomerulus.Neuron. 2002 Aug 1;35(3):412-4. doi: 10.1016/s0896-6273(02)00796-1. Neuron. 2002. PMID: 12165464 Review.
Cited by
-
The influence of single bursts versus single spikes at excitatory dendrodendritic synapses.Eur J Neurosci. 2012 Feb;35(3):389-401. doi: 10.1111/j.1460-9568.2011.07978.x. Epub 2012 Jan 25. Eur J Neurosci. 2012. PMID: 22277089 Free PMC article.
-
Cholecystokinin: an excitatory modulator of mitral/tufted cells in the mouse olfactory bulb.PLoS One. 2013 May 15;8(5):e64170. doi: 10.1371/journal.pone.0064170. Print 2013. PLoS One. 2013. PMID: 23691163 Free PMC article.
-
Optical Manipulations Reveal Strong Reciprocal Inhibition But Limited Recurrent Excitation within Olfactory Bulb Glomeruli.eNeuro. 2021 Dec 9;8(6):ENEURO.0311-21.2021. doi: 10.1523/ENEURO.0311-21.2021. Print 2021 Nov-Dec. eNeuro. 2021. PMID: 34772695 Free PMC article.
-
External tufted cells drive the output of olfactory bulb glomeruli.J Neurosci. 2009 Feb 18;29(7):2043-52. doi: 10.1523/JNEUROSCI.5317-08.2009. J Neurosci. 2009. PMID: 19228958 Free PMC article.
-
Metabotropic glutamate receptors promote disinhibition of olfactory bulb glomeruli that scales with input strength.J Neurophysiol. 2015 Mar 15;113(6):1907-20. doi: 10.1152/jn.00222.2014. Epub 2014 Dec 31. J Neurophysiol. 2015. PMID: 25552635 Free PMC article.
References
-
- Ajima A, Hensch T, Kado RT, Ito M. Differential blocking action of Joro spider toxin analog on parallel fiber and climbing fiber synapses in cerebellar Purkinje cells. Neurosci Res. 1991;12:281–286. - PubMed
-
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Glomerular synaptic responses to olfactory nerve input in rat olfactory bulb slices. Neuroscience. 1997;79:425–434. - PubMed
-
- Asami T, Kagechika H, Hashimoto Y, Shudo K, Miwa A, Kawai N, Nakajima T. Acylpolyamines mimic the action of joro spider toxin (JSTX) on crustacean muscle glutamate receptors. Biomed Res. 1989;10:185–189.
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

