Quantitative structure-activity relationship (QSAR) of indoloacetamides as inhibitors of human isoprenylcysteine carboxyl methyltransferase
- PMID: 17157012
- PMCID: PMC1941685
- DOI: 10.1016/j.bmcl.2006.11.030
Quantitative structure-activity relationship (QSAR) of indoloacetamides as inhibitors of human isoprenylcysteine carboxyl methyltransferase
Abstract
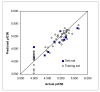
A QSAR is developed for the isoprenylcysteine carboxyl methyltransferase (ICMT) inhibitory activities of a series of indoloacetamides (n=72) that are structurally related to cysmethynil, a selective ICMT inhibitor. Multivariate analytical tools (principal component analysis (PCA) and projection to latent structures (PLS)), multi-linear regression (MLR) and comparative molecular field analysis (CoMFA) are used to develop a suitably predictive model for the purpose of optimizing and identifying members with more potent inhibitory activity. The resulting model shows that good activity is determined largely by the characteristics of the substituent attached to the indole nitrogen, which should be a lipophilic residue with fairly wide dimensions. In contrast, the substituted phenyl ring attached to the indole ring must be of limited dimensions and lipophilicity.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

