Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity
- PMID: 17157332
- PMCID: PMC1850990
- DOI: 10.1016/j.mrfmmm.2006.11.021
Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity
Abstract
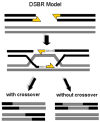
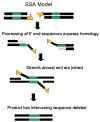
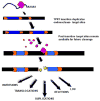
The ubiquity of mobile elements in mammalian genomes poses considerable challenges for the maintenance of genome integrity. The predisposition of mobile elements towards participation in genomic rearrangements is largely a consequence of their interspersed homologous nature. As tracts of nonallelic sequence homology, they have the potential to interact in a disruptive manner during both meiotic recombination and DNA repair processes, resulting in genomic alterations ranging from deletions and duplications to large-scale chromosomal rearrangements. Although the deleterious effects of transposable element (TE) insertion events have been extensively documented, it is arguably through post-insertion genomic instability that they pose the greatest hazard to their host genomes. Despite the periodic generation of important evolutionary innovations, genomic alterations involving TE sequences are far more frequently neutral or deleterious in nature. The potentially negative consequences of this instability are perhaps best illustrated by the >25 human genetic diseases that are attributable to TE-mediated rearrangements. Some of these rearrangements, such as those involving the MLL locus in leukemia and the LDL receptor in familial hypercholesterolemia, represent recurrent mutations that have independently arisen multiple times in human populations. While TE-instability has been a potent force in shaping eukaryotic genomes and a significant source of genetic disease, much concerning the mechanisms governing the frequency and variety of these events remains to be clarified. Here we survey the current state of knowledge regarding the mechanisms underlying mobile element-based genetic instability in mammals. Compared to simpler eukaryotic systems, mammalian cells appear to have several modifications to their DNA-repair ensemble that allow them to better cope with the large amount of interspersed homology that has been generated by TEs. In addition to the disruptive potential of nonallelic sequence homology, we also consider recent evidence suggesting that the endonuclease products of TEs may also play a key role in instigating mammalian genomic instability.
Figures


References
-
- Callinan PA, Batzer MA. In: Retrotransposable Elemenets and Human Disease. J-N V, editor. Genome Dynamics; Karger, Basel: 2006. pp. 104–115. - PubMed
-
- Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab. 1999;67:183–193. - PubMed
-
- Petrov DA, Aminetzach YT, Davis JC, Bensasson D, Hirsh AE. Size matters: non-LTR retrotransposable elements and ectopic recombination in Drosophila. Mol Biol Evol. 2003;20:880–892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

