Herpes simplex virus type 2-mediated disease is reduced in mice lacking RNase L
- PMID: 17157346
- PMCID: PMC1876699
- DOI: 10.1016/j.virol.2006.10.042
Herpes simplex virus type 2-mediated disease is reduced in mice lacking RNase L
Abstract
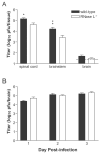
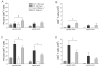
RNase L helps mediate the antiviral state induced by type I interferons (IFNalphabeta). Although herpes simplex virus (HSV) encodes inhibitors of the IFNalphabeta-induced antiviral response, the IFNalphabeta system serves the body as a first line of defense against HSV. We investigated whether RNase L limits HSV-2 replication and virulence. RNaseL(-/-) and wild-type C57BL/6 mice were infected intravaginally with HSV-2 strain 333. Although initial replication in the genital epithelium was similar, mice lacking RNase L developed less severe genital and neurologic disease than wild-type mice, survived longer, and contained lower viral titers in the nervous system. CD4(+) T cell infiltration into the genital tract and spinal cord of RNase L(-/-) mice was reduced, suggesting that a restricted inflammatory response may account for reduction in disease. Thus, RNase L does not play a significant role in control of HSV-2 infection in vivo; instead, RNase L may regulate aspects of the inflammatory response that contribute to disease.
Figures



Similar articles
-
Selective ablation of virion host shutoff protein RNase activity attenuates herpes simplex virus 2 in mice.J Virol. 2008 Apr;82(7):3642-53. doi: 10.1128/JVI.02409-07. Epub 2008 Jan 30. J Virol. 2008. PMID: 18234805 Free PMC article.
-
Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness.Virology. 2004 Apr 25;322(1):158-67. doi: 10.1016/j.virol.2004.01.019. Virology. 2004. PMID: 15063125
-
Novel Role for Interleukin-17 in Enhancing Type 1 Helper T Cell Immunity in the Female Genital Tract following Mucosal Herpes Simplex Virus 2 Vaccination.J Virol. 2017 Nov 14;91(23):e01234-17. doi: 10.1128/JVI.01234-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956763 Free PMC article.
-
Counteraction of interferon-induced antiviral responses by herpes simplex viruses.Curr Top Microbiol Immunol. 2002;269:171-85. doi: 10.1007/978-3-642-59421-2_11. Curr Top Microbiol Immunol. 2002. PMID: 12224508 Review.
-
Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa.J Reprod Immunol. 2011 Mar;88(2):210-8. doi: 10.1016/j.jri.2011.01.001. Epub 2011 Feb 21. J Reprod Immunol. 2011. PMID: 21334750 Review.
Cited by
-
Selective ablation of virion host shutoff protein RNase activity attenuates herpes simplex virus 2 in mice.J Virol. 2008 Apr;82(7):3642-53. doi: 10.1128/JVI.02409-07. Epub 2008 Jan 30. J Virol. 2008. PMID: 18234805 Free PMC article.
-
Interferon-stimulated genes: roles in viral pathogenesis.Curr Opin Virol. 2014 Jun;6:40-6. doi: 10.1016/j.coviro.2014.03.006. Epub 2014 Apr 5. Curr Opin Virol. 2014. PMID: 24713352 Free PMC article. Review.
-
Viral encounters with 2',5'-oligoadenylate synthetase and RNase L during the interferon antiviral response.J Virol. 2007 Dec;81(23):12720-9. doi: 10.1128/JVI.01471-07. Epub 2007 Sep 5. J Virol. 2007. PMID: 17804500 Free PMC article. Review. No abstract available.
-
Antiviral activity of trappin-2 and elafin in vitro and in vivo against genital herpes.J Virol. 2013 Jul;87(13):7526-38. doi: 10.1128/JVI.02243-12. Epub 2013 May 1. J Virol. 2013. PMID: 23637403 Free PMC article.
-
A scientific journey through the 2-5A/RNase L system.Cytokine Growth Factor Rev. 2007 Oct-Dec;18(5-6):381-8. doi: 10.1016/j.cytogfr.2007.06.012. Epub 2007 Jul 27. Cytokine Growth Factor Rev. 2007. PMID: 17681844 Free PMC article. Review.
References
-
- Al-khatib K, Williams BR, Silverman RH, Halford W, Carr DJ. The murine double-stranded RNA-dependent protein kinase PKR and the murine 2′,5′-oligoadenylate synthetase-dependent RNase L are required for IFNβ-mediated resistance against herpes simplex virus type 1 in primary trigeminal ganglion culture. Virology. 2003;313:126–135. - PubMed
-
- Al-khatib K, Williams BR, Silverman RH, Halford W, Carr DJ. Dichotomy between survival and lytic gene expression in RNase L- and PKR-deficient mice transduced with an adenoviral vector expressing murine IFN-beta following ocular HSV-1 infection. Exp Eye Res. 2005;80:167–173. - PubMed
-
- Austin BA, James C, Silverman RH, Carr DJJ. Critical role for the oligoadenylate synthetase/RNase L pathway in response to IFN-β during acute ocular herpes simplex virus type 1 infection. J Immunol. 2005;175:1101–1106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

