Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal
- PMID: 17157356
- PMCID: PMC1819584
- DOI: 10.1016/j.mad.2006.11.025
Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal
Abstract
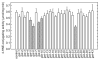
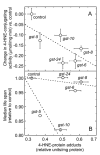
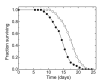
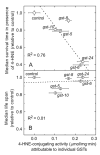
The lipid peroxidation product 4-hydroxynon-2-enal (4-HNE) forms as a consequence of oxidative stress, and acts as a signaling molecule or, at superphysiological levels, as a toxicant. The steady-state concentration of the compound reflects the balance between its generation and its metabolism, primarily through glutathione conjugation. Using an RNAi-based screen, we identified in Caenorhabditis elegans five glutathione transferases (GSTs) capable of catalyzing 4-HNE conjugation. RNAi knock-down of these GSTs (products of the gst-5, gst-6, gst-8, gst-10, and gst-24 genes) sensitized the nematode to electrophilic stress elicited by exposure to 4-HNE. However, interference with the expression of only two of these genes (gst-5 and gst-10) significantly shortened the life span of the organism. RNAi knock-down of the other GSTs resulted in at least as much 4-HNE adducts, suggesting tissue specificity of effects on longevity. Our results are consistent with the oxidative stress theory of organismal aging, broadened by considering electrophilic stress as a contributing factor. According to this extended hypothesis, peroxidation of lipids leads to the formation of 4-HNE in a chain reaction which amplifies the original damage. 4-HNE then acts as an "aging effector" via the formation of 4-HNE-protein adducts, and a resulting change in protein function.
Figures






References
-
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. - PubMed
-
- Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–270. - PubMed
-
- Ayyadevara S, Dandapat A, Singh SP, Benes H, Zimniak L, Shmookler Reis RJ, Zimniak P. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005a;4:299–307. - PubMed
-
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, Benes H, Shmookler Reis RJ, Liebau E, Zimniak P. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005b;4:257–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

