Calcium sparklets regulate local and global calcium in murine arterial smooth muscle
- PMID: 17158168
- PMCID: PMC2075382
- DOI: 10.1113/jphysiol.2006.124420
Calcium sparklets regulate local and global calcium in murine arterial smooth muscle
Abstract
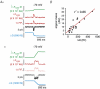
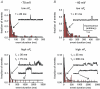
In arterial smooth muscle, protein kinase Calpha (PKCalpha) coerces discrete clusters of L-type Ca2+ channels to operate in a high open probability mode, resulting in subcellular domains of nearly continual Ca2+ influx called 'persistent Ca2+ sparklets'. Our previous work suggested that steady-state Ca2+ entry into arterial myocytes, and thus global [Ca2+]i, is regulated by Ca2+ influx through clusters of L-type Ca2+ channels operating in this persistently active mode in addition to openings of solitary channels functioning in a low-activity mode. Here, we provide the first direct evidence supporting this 'Ca2+ sparklet' model of Ca2+ influx at a physiological membrane potential and external Ca2+ concentration. In support of this model, we found that persistent Ca2+ sparklets produced local and global elevations in [Ca2+]i. Membrane depolarization increased Ca2+ influx via low-activity and high-activity persistent Ca2+ sparklets. Our data indicate that Ca2+ entering arterial smooth muscle through persistent Ca2+ sparklets accounts for approximately 50% of the total dihydropyridine-sensitive (i.e. L-type Ca2+ channel) Ca2+ influx at a physiologically relevant membrane potential (-40 mV) and external Ca2+ concentration (2 mm). Consistent with this, inhibition of basal PKCalpha-dependent persistent Ca2+ sparklets decreased [Ca2+]i by about 50% in isolated arterial myocytes and intact pressurized arteries. Taken together, these data support the conclusion that in arterial smooth muscle steady-state Ca2+ entry and global [Ca2+]i are regulated by low-activity and PKCalpha-dependent high-activity persistent Ca(2+) sparklets.
Figures




References
-
- Amberg GC, Santana LF. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. - PubMed
-
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
