STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane
- PMID: 17158173
- PMCID: PMC2151373
- DOI: 10.1113/jphysiol.2006.122432
STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane
Abstract
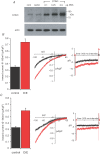
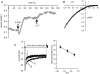
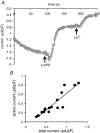
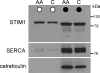
Recent studies have indicated a critical role for STIM (stromal interacting molecule) proteins in the regulation of the store-operated mode of receptor-activated Ca2+ entry. Current models emphasize the role of STIM located in the endoplasmic reticulum membrane, where a Ca2+-binding EF-hand domain within the N-terminal of the protein lies within the lumen and is thought to represent the sensor for the depletion of intracellular Ca2+ stores. Dissociation of Ca2+ from this domain induces the aggregation of STIM to regions of the ER immediately adjacent to the plasma membrane where it acts to regulate the activity of store-operated Ca2+ channels. However, the possible effects of STIM on other modes of receptor-activated Ca2+ entry have not been examined. Here we show that STIM1 also regulates the arachidonic-acid-regulated Ca2+-selective (ARC) channels - receptor-activated Ca2+ entry channels whose activation is entirely independent of store depletion. Regulation of the ARC channels by STIM1 does not involve dissociation of Ca2+ from the EF-hand, or any translocation of STIM1. Instead, a critical role of STIM1 resident in the plasma membrane is indicated. Thus, exposure of intact cells to an antibody targeting the extracellular N-terminal domain of STIM1 inhibits ARC channel activity without significantly affecting the store-operated channels. A similar specific inhibition of the ARC channels is seen in cells expressing a STIM1 construct in which the N-linked glycosylation sites essential for the constitutive cell surface expression of STIM1, were mutated. We conclude that, in contrast to store-operated channels, regulation of ARC channels by STIM1 depends exclusively on the pool of STIM1 constitutively residing in the plasma membrane. These data demonstrate that STIM1 is a more universal regulator of Ca2+ entry pathways than previously thought, and appears to have multiple modes of action.
Figures




References
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol. 2002;12:R563–R565. - PubMed
-
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

