Ceramide-activated protein phosphatase involvement in insulin resistance via Akt, serine/arginine-rich protein 40, and ribonucleic acid splicing in L6 skeletal muscle cells
- PMID: 17158207
- PMCID: PMC2664306
- DOI: 10.1210/en.2006-0750
Ceramide-activated protein phosphatase involvement in insulin resistance via Akt, serine/arginine-rich protein 40, and ribonucleic acid splicing in L6 skeletal muscle cells
Abstract
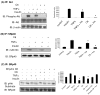
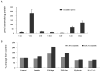
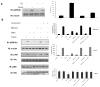
Elevated TNFalpha levels are associated with insulin resistance, but the molecular mechanisms linking cytokine signaling to impaired insulin function remain elusive. We previously demonstrated a role for Akt in insulin regulation of protein kinase CbetaII alternative splicing through phosphorylation of serine/arginine-rich protein 40, a required mechanism for insulin-stimulated glucose uptake. We hypothesized that TNFalpha attenuated insulin signaling by dephosphorylating Akt and its targets via ceramide-activated protein phosphatase. Western blot analysis of L6 cell lysates demonstrated impaired insulin-stimulated phosphorylation of Akt, serine/arginine-rich protein 40, and glycogen synthase kinase 3beta in response to TNFalpha and the short chain C6 ceramide analog. TNFalpha increased serine/threonine phosphatase activity of protein phosphatase 1 (PP1) in response to C6, but not insulin, suggesting a ceramide-specific effect. Myriocin, an inhibitor of de novo ceramide synthesis, blocked stimulation of the PP1 activity. Ceramide species measurement by liquid chromatography-mass spectrometry showed consistent increases in C24:1 and C16 ceramides. Effects of TNFalpha and C6 on insulin-stimulated phosphorylation of glycogen synthase kinase 3beta were prevented by myriocin and tautomycin, a PP1 inhibitor, further implicating a de novo ceramide-PP1 pathway. Alternative splicing assays demonstrated that TNFalpha abolished insulin-mediated inclusion of the protein kinase CbetaII exon. Collectively, our work demonstrates a role for PP1-like ceramide-activated protein phosphatase in mediating TNFalpha effects blocking insulin phosphorylation cascades involved in glycogen metabolism and alternative splicing.
Figures
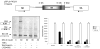
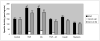
References
-
- Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes. 2002;51:1876–83. - PubMed
-
- Borst SE. The role of TNF-alpha in insulin resistance. Endocrine. 2004;23:177–82. - PubMed
-
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–7. - PubMed
-
- Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–5. - PubMed
-
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. - PubMed

