Analysis of the oxidative damage-induced conformational changes of apo- and holocalmodulin by dose-dependent protein oxidative surface mapping
- PMID: 17158574
- PMCID: PMC1796823
- DOI: 10.1529/biophysj.106.099093
Analysis of the oxidative damage-induced conformational changes of apo- and holocalmodulin by dose-dependent protein oxidative surface mapping
Abstract
Calmodulin (CaM) is known to undergo conformational and functional changes on oxidation, allowing CaM to function as an oxidative stress sensor. We report the use of a novel mass spectrometry-based methodology to monitor the structure of apo- and holo-CaM as it undergoes conformational changes as a result of increasing amounts of oxidative damage. The kinetics of oxidation for eight peptides are followed by mass spectrometry, and 12 sites of oxidation are determined by MS/MS. Changes in the pseudo-first-order rate constant of oxidation for a peptide after increasing radiation exposure reveal changes in the accessibility of the peptide to the diffusing hydroxyl radical, indicating conformational changes as a function of increased oxidative damage. For holo-CaM, most sites rapidly become less exposed to hydroxyl radicals as the protein accumulates oxidative damage, indicating a closing of the hydrophobic pockets in the N- and C-terminal lobes. For apo-CaM, many of the sites rapidly become more exposed until they resemble the solvent accessibility of holo-CaM in the native structure and then rapidly become more buried, mimicking the conformational changes of holo-CaM. At the most heavily damaged points measured, the rates of oxidation for both apo- and holo-CaM are essentially identical, suggesting the two assume similar structures.
Figures


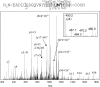
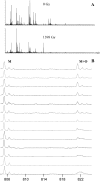

References
-
- Yap, K. L., J. Kim, K. Truong, M. Sherman, T. Yuan, and M. Ikura. 2000. Calmodulin target database. J. Struct. Funct. Genomics. 1:8–14. - PubMed
-
- Babu, Y. S., C. E. Bugg, and W. J. Cook. 1988. Structure of calmodulin refined at 2.2 A resolution. J. Mol. Biol. 204:191–204. - PubMed
-
- Chattopadhyaya, R., W. E. Meador, A. R. Means, and F. A. Quiocho. 1992. Calmodulin structure refined at 1.7 A resolution. J. Mol. Biol. 228:1177–1192. - PubMed
-
- Barbato, G., M. Ikura, L. E. Kay, R. W. Pastor, and A. Bax. 1992. Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible. Biochemistry. 31:5269–5278. - PubMed
-
- Zhang, M., T. Tanaka, and M. Ikura. 1995. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 2:758–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

