Stimulus perception in bacterial signal-transducing histidine kinases
- PMID: 17158704
- PMCID: PMC1698512
- DOI: 10.1128/MMBR.00020-06
Stimulus perception in bacterial signal-transducing histidine kinases
Abstract
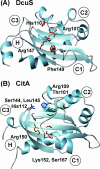
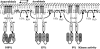
Two-component signal-transducing systems are ubiquitously distributed communication interfaces in bacteria. They consist of a histidine kinase that senses a specific environmental stimulus and a cognate response regulator that mediates the cellular response, mostly through differential expression of target genes. Histidine kinases are typically transmembrane proteins harboring at least two domains: an input (or sensor) domain and a cytoplasmic transmitter (or kinase) domain. They can be identified and classified by virtue of their conserved cytoplasmic kinase domains. In contrast, the sensor domains are highly variable, reflecting the plethora of different signals and modes of sensing. In order to gain insight into the mechanisms of stimulus perception by bacterial histidine kinases, we here survey sensor domain architecture and topology within the bacterial membrane, functional aspects related to this topology, and sequence and phylogenetic conservation. Based on these criteria, three groups of histidine kinases can be differentiated. (i) Periplasmic-sensing histidine kinases detect their stimuli (often small solutes) through an extracellular input domain. (ii) Histidine kinases with sensing mechanisms linked to the transmembrane regions detect stimuli (usually membrane-associated stimuli, such as ionic strength, osmolarity, turgor, or functional state of the cell envelope) via their membrane-spanning segments and sometimes via additional short extracellular loops. (iii) Cytoplasmic-sensing histidine kinases (either membrane anchored or soluble) detect cellular or diffusible signals reporting the metabolic or developmental state of the cell. This review provides an overview of mechanisms of stimulus perception for members of all three groups of bacterial signal-transducing histidine kinases.
Figures

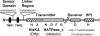




References
-
- Abada, E. M., A. Balzer, A. Jager, and G. Klug. 2002. Bacteriochlorophyll-dependent expression of genes for pigment-binding proteins in Rhodobacter capsulatus involves the RegB/RegA two-component system. Mol. Genet. Genomics 267:202-209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

