A dynamic, mitotic-like mechanism for bacterial chromosome segregation
- PMID: 17158745
- PMCID: PMC1686604
- DOI: 10.1101/gad.1496506
A dynamic, mitotic-like mechanism for bacterial chromosome segregation
Abstract
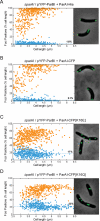
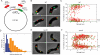
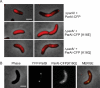
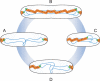
The mechanisms that mediate chromosome segregation in bacteria are poorly understood. Despite evidence of dynamic movement of chromosome regions, to date, mitotic-like mechanisms that act on the bacterial chromosome have not been demonstrated. Here we provide evidence that the Vibrio cholerae ParAI and ParBI proteins are components of an apparatus that pulls the origin region of the large V. cholerae chromosome to the cell pole and anchors it there. ParBI interacts with a conserved origin-proximal, centromere-like site (parSI) that, following chromosome replication, segregates asymmetrically from one pole to the other. While segregating, parSI stretches far away from neighboring chromosomal loci. ParAI forms a dynamic band that extends from the pole to the segregating ParBI/parSI complex. Movement of ParBI/parSI across the cell occurs in concert with ParAI retraction. Deletion of parAI disrupts proper origin localization and segregation dynamics, and parSI no longer separates from nearby regions. These data suggest that ParAI forms a dynamic structure that pulls the ParBI-bound chromosome to the pole in a process analogous to anaphase of eukaryotic mitosis.
Figures



References
-
- Adachi, S., Hori, K., Hiraga, S. Subcellular positioning of F plasmid mediated by dynamic localization of SopA and SopB. J. Mol. Biol. 2006;356:850–863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
