Hypoglycemia leads to age-related loss of vision
- PMID: 17159157
- PMCID: PMC1697832
- DOI: 10.1073/pnas.0604478104
Hypoglycemia leads to age-related loss of vision
Abstract
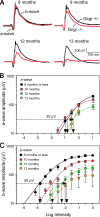
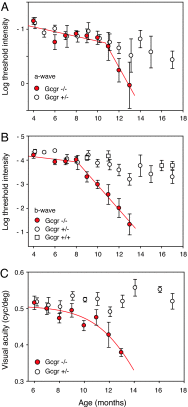
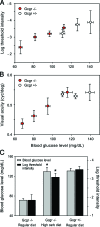
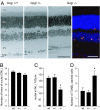
The retina is among the most metabolically active tissues in the body, requiring a constant supply of blood glucose to sustain function. We assessed the impact of low blood glucose on the vision of C57BL/6J mice rendered hypoglycemic by a null mutation of the glucagon receptor gene, Gcgr. Metabolic stress from moderate hypoglycemia led to late-onset loss of retinal function in Gcgr(-/-) mice, loss of visual acuity, and eventual death of retinal cells. Retinal function measured by the electroretinogram b-wave threshold declined >100-fold from age 9 to 13 months, whereas decreases in photoreceptor function measured by the ERG a-wave were delayed by 3 months. At 10 months of age Gcgr(-/-) mice began to lose visual acuity and exhibit changes in retinal anatomy, including an increase in cell death that was initially more pronounced in the inner retina. Decreases in retinal function and visual acuity correlated directly with the degree of hypoglycemia. This work demonstrates a metabolic-stress-induced loss of vision in mammals, which has not been described previously. Linkage between low blood glucose and loss of vision in mice may highlight the importance for glycemic control in diabetics and retinal diseases related to metabolic stress as macular degeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- McFarland RA, Halperin MH, Niven JI. Am J Physiol. 1945;144:378–388.
-
- Tabandeh H, Ranganath L, Marks V. Eur J Ophthalmol. 1996;6:81–86. - PubMed
-
- McGrimmon RJ, Deary IJ, Huntly BJP, MacLeod KJ, Frier BM. Brain. 1996;119:1277–1287. - PubMed
-
- Clore J, Nestler J, Blackard W. Diabetes. 1989;38:285–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

