H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability
- PMID: 17159999
- PMCID: PMC2819265
- DOI: 10.1038/ncb1514
H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability
Abstract
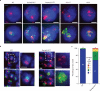
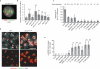
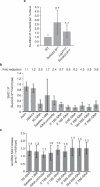
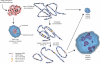
Investigations aimed at identifying regulators of nuclear architecture in Drosophila demonstrated that cells lacking H3K9 methylation and RNA interference (RNAi) pathway components displayed disorganized nucleoli, ribosomal DNA (rDNA) and satellite DNAs. The levels of H3K9 dimethylation (H3K9me2) in chromatin associated with repeated DNAs decreased dramatically in Su(var)3-9 and dcr-2 (dicer-2) mutant tissues compared with wild type. We also observed a substantial increase in extrachromosomal circular (ecc) repeated DNAs in mutant tissues. The disorganized nucleolus phenotype depends on the presence of Ligase 4 and ecc DNA formation is not induced by removal of cohesin. We conclude that the structural integrity and organization of repeated DNAs and nucleoli are regulated by the H3K9 methylation and RNAi pathways, and other regulators of heterochromatin-mediated silencing. In addition, repeated DNA stability involves suppression of non-homologous end joining (NHEJ) or other recombination pathways. These results suggest a mechanism for how local chromatin structure can regulate genome stability, and the organization of chromosomal elements and nuclear organelles.
Figures




Comment in
-
Heterochromatin: condense or excise.Nat Cell Biol. 2007 Jan;9(1):19-20. doi: 10.1038/ncb0107-19. Nat Cell Biol. 2007. PMID: 17199128 No abstract available.
Similar articles
-
Heterochromatin: condense or excise.Nat Cell Biol. 2007 Jan;9(1):19-20. doi: 10.1038/ncb0107-19. Nat Cell Biol. 2007. PMID: 17199128 No abstract available.
-
Loss of nucleolar histone chaperone NPM1 triggers rearrangement of heterochromatin and synergizes with a deficiency in DNA methyltransferase DNMT3A to drive ribosomal DNA transcription.J Biol Chem. 2014 Dec 12;289(50):34601-19. doi: 10.1074/jbc.M114.569244. Epub 2014 Oct 27. J Biol Chem. 2014. PMID: 25349213 Free PMC article.
-
Histone modification and the control of heterochromatic gene silencing in Drosophila.Chromosome Res. 2006;14(4):377-92. doi: 10.1007/s10577-006-1066-1. Chromosome Res. 2006. PMID: 16821134 Review.
-
Lysine 27 dimethylation of Drosophila linker histone dH1 contributes to heterochromatin organization independently of H3K9 methylation.Nucleic Acids Res. 2022 Sep 9;50(16):9212-9225. doi: 10.1093/nar/gkac716. Nucleic Acids Res. 2022. PMID: 36039761 Free PMC article.
-
Heterochromatin: RNA points the way.Curr Biol. 2004 Sep 21;14(18):R759-61. doi: 10.1016/j.cub.2004.09.014. Curr Biol. 2004. PMID: 15380087 Review.
Cited by
-
A Glimpse of "Dicer Biology" Through the Structural and Functional Perspective.Front Mol Biosci. 2021 May 7;8:643657. doi: 10.3389/fmolb.2021.643657. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026825 Free PMC article. Review.
-
Formation of nuclear heterochromatin: the nucleolar point of view.Epigenetics. 2012 Aug;7(8):811-4. doi: 10.4161/epi.21072. Epub 2012 Jun 27. Epigenetics. 2012. PMID: 22735386 Free PMC article. Review.
-
Unique Epigenetic Features of Ribosomal RNA Genes (rDNA) in Early Diverging Plants (Bryophytes).Front Plant Sci. 2019 Sep 5;10:1066. doi: 10.3389/fpls.2019.01066. eCollection 2019. Front Plant Sci. 2019. PMID: 31543890 Free PMC article.
-
Replication fork stalling elicits chromatin compaction for the stability of stalling replication forks.Proc Natl Acad Sci U S A. 2019 Jul 16;116(29):14563-14572. doi: 10.1073/pnas.1821475116. Epub 2019 Jul 1. Proc Natl Acad Sci U S A. 2019. PMID: 31262821 Free PMC article.
-
Nuclear dynamics of radiation-induced foci in euchromatin and heterochromatin.Mutat Res. 2013 Oct;750(1-2):56-66. doi: 10.1016/j.mrfmmm.2013.08.001. Epub 2013 Aug 16. Mutat Res. 2013. PMID: 23958412 Free PMC article. Review.
References
-
- Francastel C, Schubeler D, Martin DI, Groudine M. Nuclear compartmentalization and gene activity. Nature Rev. Mol. Cell Biol. 2000;1:137–143. - PubMed
-
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nature Rev. Genet. 2001;2:292–301. - PubMed
-
- John B. The biology of heterochromatin. In: Verma RS, editor. Heterochromatin: Molecular and Structural Aspects. Cambridge University Press; Cambridge: 1988. pp. 1–147.
-
- Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nature Rev. Mol. Cell Biol. 2005;6:838–849. - PubMed
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

