Three drug combinations for late-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Uganda
- PMID: 17160135
- PMCID: PMC1687208
- DOI: 10.1371/journal.pctr.0010039
Three drug combinations for late-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Uganda
Abstract
Objectives: Our objective was to compare the efficacy and safety of three drug combinations for the treatment of late-stage human African trypanosomiasis caused by Trypanosoma brucei gambiense.
Design: This trial was a randomized, open-label, active control, parallel clinical trial comparing three arms.
Setting: The study took place at the Sleeping Sickness Treatment Center run by Médecins Sans Frontières at Omugo, Arua District, Uganda
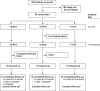
Participants: Stage 2 patients diagnosed in Northern Uganda were screened for inclusion and a total of 54 selected.
Interventions: Three drug combinations were given to randomly assigned patients: melarsoprol-nifurtimox (M+N), melarsoprol-eflornithine (M+E), and nifurtimox-eflornithine (N+E). Dosages were uniform: intravenous (IV) melarsoprol 1.8 mg/kg/d, daily for 10 d; IV eflornithine 400 mg/kg/d, every 6 h for 7 d; oral nifurtimox 15 (adults) or 20 (children <15 y) mg/kg/d, every 8 h for 10 d. Patients were followed up for 24 mo.
Outcome measures: Outcomes were cure rates and adverse events attributable to treatment.
Results: Randomization was performed on 54 patients before enrollment was suspended due to unacceptable toxicity in one of the three arms. Cure rates obtained with the intention to treat analysis were M+N 44.4%, M+E 78.9%, and N+E 94.1%, and were significantly higher with N+E (p = 0.003) and M+E (p = 0.045) than with M+N. Adverse events were less frequent and less severe with N+E, resulting in fewer treatment interruptions and no fatalities. Four patients died who were taking melarsoprol-nifurtimox and one who was taking melarsoprol-eflornithine.
Conclusions: The N+E combination appears to be a promising first-line therapy that may improve treatment of sleeping sickness, although the results from this interrupted study do not permit conclusive interpretations. Larger studies are needed to continue the evaluation of this drug combination in the treatment of T. b. gambiense sleeping sickness.
Conflict of interest statement
Figures
Similar articles
-
Nifurtimox-eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo.Clin Infect Dis. 2007 Dec 1;45(11):1435-42. doi: 10.1086/522982. Epub 2007 Oct 22. Clin Infect Dis. 2007. PMID: 17990225 Clinical Trial.
-
Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial.Lancet. 2009 Jul 4;374(9683):56-64. doi: 10.1016/S0140-6736(09)61117-X. Epub 2009 Jun 24. Lancet. 2009. PMID: 19559476 Clinical Trial.
-
Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial.Lancet. 2018 Jan 13;391(10116):144-154. doi: 10.1016/S0140-6736(17)32758-7. Epub 2017 Nov 4. Lancet. 2018. PMID: 29113731 Clinical Trial.
-
Efficacy and Toxicity of Fexinidazole and Nifurtimox Plus Eflornithine in the Treatment of African Trypanosomiasis: A Systematic Review.Cureus. 2021 Aug 4;13(8):e16881. doi: 10.7759/cureus.16881. eCollection 2021 Aug. Cureus. 2021. PMID: 34513456 Free PMC article. Review.
-
Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness).Lancet Neurol. 2013 Feb;12(2):186-94. doi: 10.1016/S1474-4422(12)70296-X. Epub 2012 Dec 21. Lancet Neurol. 2013. PMID: 23260189 Review.
Cited by
-
Cardiac involvement with parasitic infections.Clin Microbiol Rev. 2010 Apr;23(2):324-49. doi: 10.1128/CMR.00054-09. Clin Microbiol Rev. 2010. PMID: 20375355 Free PMC article. Review.
-
The development of drugs for treatment of sleeping sickness: a historical review.Parasit Vectors. 2010 Mar 10;3(1):15. doi: 10.1186/1756-3305-3-15. Parasit Vectors. 2010. PMID: 20219092 Free PMC article.
-
Eliminating human African trypanosomiasis: where do we stand and what comes next?PLoS Med. 2008 Feb;5(2):e55. doi: 10.1371/journal.pmed.0050055. PLoS Med. 2008. PMID: 18303943 Free PMC article. Review.
-
High failure rates of melarsoprol for sleeping sickness, Democratic Republic of Congo.Emerg Infect Dis. 2008 Jun;14(6):966-7. doi: 10.3201/eid1406.071266. Emerg Infect Dis. 2008. PMID: 18507916 Free PMC article.
-
A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda.Parasit Vectors. 2018 Feb 22;11(1):105. doi: 10.1186/s13071-018-2634-x. Parasit Vectors. 2018. PMID: 29471865 Free PMC article. Clinical Trial.
References
-
- Pepin J, Milord F, Khonde AN, Niyonsenga T, Loko L, et al. Risk factors for encephalopathy and mortality during melarsoprol treatment of Trypanosoma brucei gambiense sleeping sickness. Trans R Soc Trop Med Hyg. 1995;89:92–97. - PubMed
-
- World Health Organization. WHO Technical Report Series 881. Geneva: WHO; 1998. Control and surveillance of African trypanosomiasis: Report of a WHO expert committee.120 - PubMed
-
- Burri C, Keiser J. Pharmacokinetic investigations in patients from northern Angola refractory to melarsoprol treatment. Trop Med Int Health. 2001;6:412–420. - PubMed
-
- Legros D, Evans S, Maiso F, Enyaru JC, Mbulamberi D. Risk factors for treatment failure after melarsoprol for Trypanosoma brucei gambiense trypanosomiasis in Uganda. Trans R Soc Trop Med Hyg. 1999;93:439–442. - PubMed
-
- Stanghellini A, Josenando T. The situation of sleeping sickness in Angola: A calamity. Trop Med Int Health. 2001;6:330–334. - PubMed
LinkOut - more resources
Full Text Sources
Medical

